1. Plutonium-239 is used as the energy source for heart pacemakers and space probes. It decays by alpha emission. Calculate Am in grams when 1 mole of Pu-239 decays. How much energy in (kilojoules) is given off by the decay of 2 mg of Pu-239?
1. Plutonium-239 is used as the energy source for heart pacemakers and space probes. It decays by alpha emission. Calculate Am in grams when 1 mole of Pu-239 decays. How much energy in (kilojoules) is given off by the decay of 2 mg of Pu-239?
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter14: Nuclear Chemistry
Section: Chapter Questions
Problem 14.98PAE
Related questions
Question
KINDLY WRITE IN GOOD PENMANSHIP PLEASE BECAUSE SOMETIMES I CAN'T UNDERSTAND THE WRITINGS.
ACT F-1
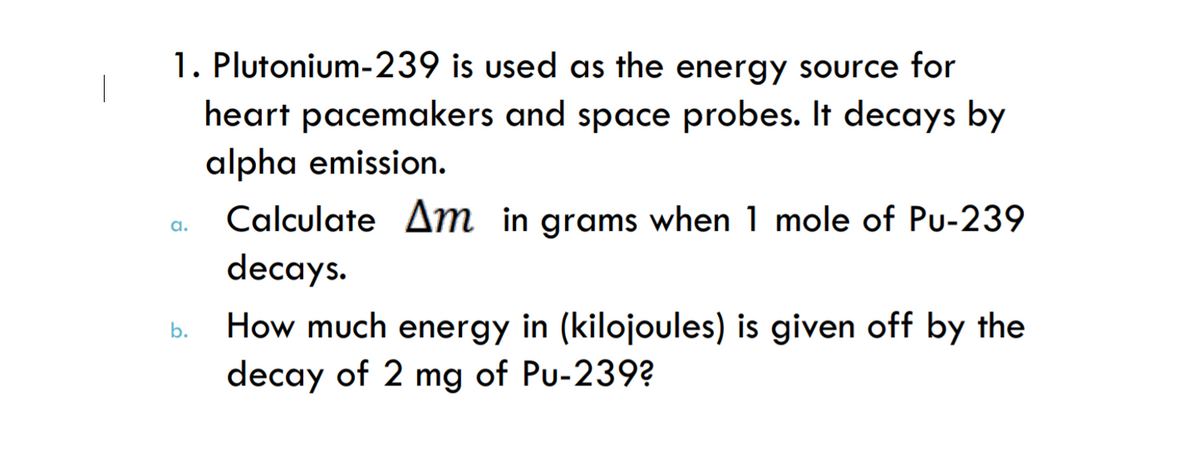
Transcribed Image Text:1. Plutonium-239 is used as the energy source for
heart pacemakers and space probes. It decays by
alpha emission.
Calculate Am in grams when 1 mole of Pu-239
a.
decays.
How much energy in (kilojoules) is given off by the
decay of 2 mg of Pu-239?
b.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 1 images

Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning