1. Shown is the structure of 1,3-butadiene, a chemical used to make plastics. How many sigma (0) and pi (m) bonds are present in 1,3-butadiene? H H H A. 7 o bonds, 2 n bonds B. 2 o bonds, 7 t bonds C. 9 o bonds, 2 1 bonds D. 11 o bonds, 2 r bonds 2. Which of the following is FALSE regarding Valence Bond Theory (VBT)? A. The formation of a covalent bond arises from the overlap of atomic orbitals. B. Bond strength increases as the distance between atoms decreases indefinitely. C. Hybrid orbitals differ in shape as the atomic orbitals they are mixed from. D. The total number of hybrid orbitals formed equals the sum of all atomic orbitals in a molecule. 3. Using the valence-bond model, what electron-group geometry is denoted by an sp hybridization? A. Linear B. Trigonal planar C. Trigonal pyramidal D. Tetrahedral
1. Shown is the structure of 1,3-butadiene, a chemical used to make plastics. How many sigma (0) and pi (m) bonds are present in 1,3-butadiene? H H H A. 7 o bonds, 2 n bonds B. 2 o bonds, 7 t bonds C. 9 o bonds, 2 1 bonds D. 11 o bonds, 2 r bonds 2. Which of the following is FALSE regarding Valence Bond Theory (VBT)? A. The formation of a covalent bond arises from the overlap of atomic orbitals. B. Bond strength increases as the distance between atoms decreases indefinitely. C. Hybrid orbitals differ in shape as the atomic orbitals they are mixed from. D. The total number of hybrid orbitals formed equals the sum of all atomic orbitals in a molecule. 3. Using the valence-bond model, what electron-group geometry is denoted by an sp hybridization? A. Linear B. Trigonal planar C. Trigonal pyramidal D. Tetrahedral
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter4: Molecular Structure And Orbitals
Section: Chapter Questions
Problem 5RQ: What hybridization is required for central atoms that have a tetrahedral arrangement of electron...
Related questions
Question
answer all please
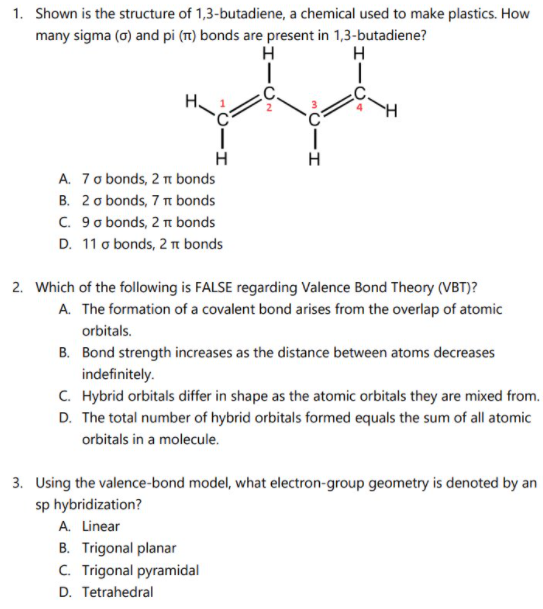
Transcribed Image Text:1. Shown is the structure of 1,3-butadiene, a chemical used to make plastics. How
many sigma (a) and pi (T) bonds are present in 1,3-butadiene?
H
H
H
H
A. 7 o bonds, 2 n bonds
B. 2 o bonds, 7 t bonds
C. 9o bonds, 2 nt bonds
D. 11 o bonds, 2 n bonds
2. Which of the following is FALSE regarding Valence Bond Theory (VBT)?
A. The formation of a covalent bond arises from the overlap of atomic
orbitals.
B. Bond strength increases as the distance between atoms decreases
indefinitely.
C. Hybrid orbitals differ in shape as the atomic orbitals they are mixed from.
D. The total number of hybrid orbitals formed equals the sum of all atomic
orbitals in a molecule.
3. Using the valence-bond model, what electron-group geometry is denoted by an
sp hybridization?
A. Linear
B. Trigonal planar
C. Trigonal pyramidal
D. Tetrahedral
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax