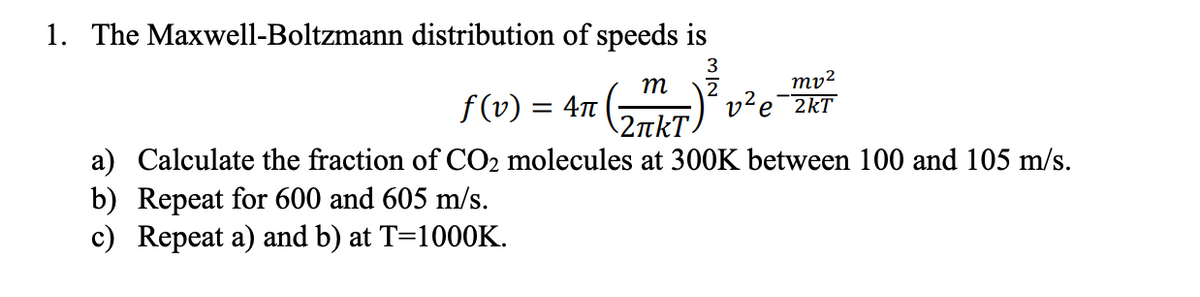
1. The Maxwell-Boltzmann distribution of speeds is 3 m mv² f (v) = 4T 2 v²e 2kT \2tkT) a) Calculate the fraction of CO2 molecules at 300K between 100 and 105 m/s. b) Repeat for 600 and 605 m/s. c) Repeat a) and b) at T=1000K.
Q: Simulate the motion of an idealized projectile in PhysLab. What is the range of the shuttlecock laun...
A: Given: The initial speed is 5.5306 m/s Height is 2.1021 m Magnitude is 9.4691 m/s2 To find the range...
Q: An edge-on spectroscopic binary is monitored throughout its orbit. The spectroscopy indicates the or...
A: In binary stars, both stars exerts gravitational force on each other, thus they rotate around a comm...
Q: COLLAB) Simulate projectile motion with air resistance in PhysLab. A shuttlecock is launched from th...
A:
Q: Find the voltage every were provided that the boundary condition is given in the graph below V= V, S...
A: To find voltage everywhere we just have to integrate the given function with limits as the boundary ...
Q: O. 8: A van is moving with a speed of 108 km/hr on level road where coefficient of friction between ...
A: To find-Radius (r)=?Given-Speed of car (V)=108 km/hrV=108×10003600m/sV=30 m/sCoefficient of friction...
Q: 6- prove that average (a + a*)² equal to (2n + 1) and the average of (a – a*)² is –(2n + 1)
A: Given: To prove that average (a+a+)2 = (2n+1) and the average of (a-a+)2 is -(2n+1)
Q: 5. Determine the amount of shearing stress develop in a material when a force of 60N is applied to a...
A: Given, Force F = 60 N Area A = 5 m2 The shearing stress can be calculated by ...
Q: Problem 2.5 A particle in the infinite square well has as its initial wave function an even mixture ...
A: Solution: (a). The given wavefunction can be normalized as the following: ψx,0ψx,0=1A2<ψ1x+<ψ2...
Q: 5- prove that average of X3 equal to zero , which (n|X|n >= (n|X³]n >= 0 , where 3 x³ = 2mw) ħ 17 (а...
A: Given:
Q: A width of 50 micron LDPE film with a density 0.925g/cm3 is required for producing plastic bags. The...
A:
Q: Ex. 85: A black body at 1000°C radiates 10 watts per sq: cm of its surface. The solar surface radiat...
A: Stefan-Boltzmann law:The total emission rate or emissive power of a blackbody are proportional to th...
Q: Q. 29: A wire of length (L) and radius (r) is loaded with a weight (Mg). If (Y) and (o) denote the Y...
A:
Q: *3-44. A scale is constructed using the 10-kg mass, the 2-kg pan P, and the pulley and cord arrangem...
A:
Q: qual charges, q, are situated at the corners of a reg e on each numeral of a clock face). What is th...
A: d) Given: 13 sided pentagon with charge q on 12 corners.
Q: Drone Parcel +y Drone time=0:Take off! Parcel time= 8.00s: Percel relese! trajtctor of Parcel from d...
A: Its a very simple but a confusing question. To answer this question we have to read the question at ...
Q: At what altitude would a geostationary sattelite need to be above the surface of Mars? Assume the ma...
A: A satellite in a geostationary orbit will move in the same direction and with the same period around...
Q: Astronomers observe a small moon of a planet and find that its average orbital radius is 5.67 x10^8 ...
A: Kepler's first law states that planets move around the sun in elliptical orbits with the sun at one ...
Q: A car is stopped at a traffic light. It then travels along a straight road so that its distance from...
A:
Q: We will use a three-wheeled cart on a flat and smooth table. A string is attached to the front of th...
A: A push or pull that an object produces on other things is known as force. Because acceleration is th...
Q: Problem 2.7 A particle in the infinite square well has the initial wave function 0 < x < a/2, JA (a ...
A: Quantum mechanics shows the nature of the motion of a microscopic particle. The behaviour of quantum...
Q: Question 4 %3D What is the differential element for the line deifned by r = 5 and 0 = 90°? O Ar de ê...
A: Given, r=5θ=900
Q: The weight of the rock in the air is 4.5 N. When it is completely submerged in water, its weight is ...
A: Given that, Weight of rock in air = 4.5 N weight of rock in water = 2 N
Q: A piece of cork, mass (8.200x10^1) g, and density (4.60x10^2) kg/m is completely immersed in water o...
A: Archimedes’ principle states that the buoyant force on an object equals the weight of the fluid it d...
Q: 1- (Exercise) Given the vectors à = Tì-2), B = ì-3Ê, and C = 2î-23+3Â, calculate the fol- (1) lowing...
A: Since we answer up to 3 subparts we will answer the first three parts only. Please resubmit the ques...
Q: Title Acceleration deviation (8x)? Trial # (i) (m/s?) (ба, —а, -а) 1 -0.836 -0.806 -0.821 4 -0.891 -...
A: We need to find the mean of the data given above. The mean of a variable x is given by X¯=X1+X2+X3+....
Q: Q10 The planet Saturn has a mass of 5.70 x 1026 kg and a diameter of 139820 km. A satellite in orbit...
A:
Q: Using an Example LED computation, What Value series resistor should be used to produce 21mA of LED, ...
A: Given,Current I=21mARs=1000 ohmVs=15VVd=2.5V Note : We’ll answer the first question since the exact ...
Q: 3 - Given a scalar field T = (x +3)y² sin(2z) and a vector field Ä = ryi + y²(z+5)k, calculate (3) t...
A: The gradient function is given by: ∇⇀=i^∂∂x+j^∂∂y+k^∂∂z Thus, we will use this expression to solve t...
Q: QUESTION 1 What is the hole quasi-Fermi level w.r.t. intrinsic level for silicon crystal at 300K dop...
A:
Q: In the methane molecule, CH4, each hydrogen atom is at the corner of a regular tetrahedron with the ...
A: This problem can be solved using the formula for dot product of two vectors.
Q: How we get this formula: 2n, n2 by using these relations: hz ni mz= (1-n2T) (1-nizł)
A: The problem can be solved by using the relation , at first we need to add the terms and then using t...
Q: Problem 2.1 (a) Twelve equal charges, q, are situated at the corners of a regular 12-sided polygon (...
A: Given, Charge are situated at the corner of the polygen.
Q: An ambulance with a siren emitting a whine at 1750 Hz overtakes and passes a cyclist pedaling a bike...
A: Given Source frequency fsource = 1750 Hz Observed frequency fobs = 1739 Observer velocity vobs = ...
Q: Use the figure below to answer the following questions: 1. If figure below represents a standing wa...
A:
Q: A frictionless object with mass m, is connected to two springs with spring constants k, and k2 respe...
A:
Q: 8-Kj mass and a 0.5 meters beyond damping constani= 15 Spring witn can be Shezhed its natory! length...
A: The mass is given as, m=8 kg The damping constant is given as, c=15 kg/s The force applied is given ...
Q: Which shows the relationship between the centripetal force acting on an. orbiting satellite and its ...
A: The rate of change of displacement is known as velocity. Instantaneous velocity is given as: v=dsdt ...
Q: 810 N B 580 58° A, BD A,
A:
Q: A small electric immersion heater is used to heat 65 g of water for a cup of instant coffee. The hea...
A:
Q: The concept of escape velocity can be best described as: (A) The initial speed required so that an o...
A: The escape velocity of a planet is the velocity required for the object to escape from the gravitati...
Q: An object is dropped from a certain height many times. For each trial, the time of flight for this o...
A: Uncertainty in the measured time value is ∆t=uncertainty in the time measurement =smallest incre...
Q: В I 1 А R D I 2 R ww E Fig. 4. Three identical resistors in a circuit. 6. Take a clockwise path A→B→...
A: Kirchhoff's voltage law states that the sum of all emf's around a closed loop must be zero. Consider...
Q: The Keck Telescope (on Mauna Kea, Hawaii) has an aperture D = 10.0 m. Its Cassegrain focus has f/15....
A:
Q: If the free end of the pulley rope is pulled down with an acceleration of 4 ft/s2, what will the acc...
A:
Q: Q9 A Physics teacher is teaching a class about the effect of friction. They assemble a track that ha...
A: Given: m=0.05kg, r=0.3m, h=1m The potential energy of the toy car at the given initial height is : ...
Q: A tiger leaps horizontally from a 15-m high cliff to reach an 8-m high rock, 15m away. (a) With a ta...
A:
Q: Two particles move about each other in circular orbits under the influence of gravitational forces, ...
A: The gravitational force between two particles is directly proportional to the product of their masse...
Q: Prove that d -e² = e² dz Hint: expansion e? with taylor series.
A:
Q: 3-89 Saturated water vapor at 350°C is heated at constant pressure until its volume has doubled. Det...
A:
Q: system using the indicated generalized coondinates. R Figare (1) Figure (2)
A: Given:
This is a
Trending now
This is a popular solution!
Step by step
Solved in 3 steps
