1. The true value of a particular measurement is 131.0 µg/L. Four chemists (A, B, C and D) each repeat the same procedure six times. The individual values obtained are displayed in the table below. Comment on the results of each chemist in terms of accuracy and precision (use standard deviation as in indicator of precision and % error as a measure of accuracy). Chemist A 130.7 131.6 133.5 132.3 132.6 129.1 Chemist B Chemist C Chemist D 125.0 132.3 136.9 137.9 125.9 131.6 136.7 134.5 134.1 135.4 136.0 137.6 130.7 109.9 131.9 115.6 131.3 132.6 2. Supposing that two chemists (A and B) in item #1 above, used two different apparatus. In order to determine if the precision of these apparatus is significantly different, apply the F-test. 3. Return to the data of chemists A and C in item #1 above. If the true value is 131.0 µg/L, calculate and compare the respective values of parameter t for these chemists. Can the presence of a systematic error be concluded for the data of either chemist A or chemist C? 4. The results of experimental measurements on %SO, in a sample repeated five times were the following: 24.24, 24.36, 24.97, 24.20, 24.10 Verify whether the third value, which seems to be high compared to the others, should be considered as an outlier. Use the Grubbs Test at 95% confidence level.
1. The true value of a particular measurement is 131.0 µg/L. Four chemists (A, B, C and D) each repeat the same procedure six times. The individual values obtained are displayed in the table below. Comment on the results of each chemist in terms of accuracy and precision (use standard deviation as in indicator of precision and % error as a measure of accuracy). Chemist A 130.7 131.6 133.5 132.3 132.6 129.1 Chemist B Chemist C Chemist D 125.0 132.3 136.9 137.9 125.9 131.6 136.7 134.5 134.1 135.4 136.0 137.6 130.7 109.9 131.9 115.6 131.3 132.6 2. Supposing that two chemists (A and B) in item #1 above, used two different apparatus. In order to determine if the precision of these apparatus is significantly different, apply the F-test. 3. Return to the data of chemists A and C in item #1 above. If the true value is 131.0 µg/L, calculate and compare the respective values of parameter t for these chemists. Can the presence of a systematic error be concluded for the data of either chemist A or chemist C? 4. The results of experimental measurements on %SO, in a sample repeated five times were the following: 24.24, 24.36, 24.97, 24.20, 24.10 Verify whether the third value, which seems to be high compared to the others, should be considered as an outlier. Use the Grubbs Test at 95% confidence level.
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 100AE: The active ingredient of aspirin tablets is acetylsalicylic acid, which, has a density of 1.4 g/cm3....
Related questions
Question
number 3 and 4 of this part ty in analytical chem
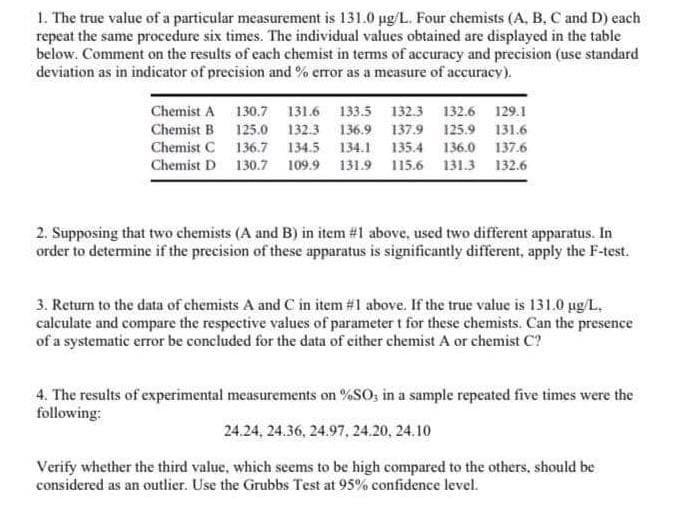
Transcribed Image Text:1. The true value of a particular measurement is 131.0 µg/L. Four chemists (A, B, C and D) each
repeat the same procedure six times. The individual values obtained are displayed in the table
below. Comment on the results of each chemist in terms of accuracy and precision (use standard
deviation as in indicator of precision and % error as a measure of accuracy).
Chemist A
Chemist B
Chemist C
Chemist D
130.7 131.6 133.5 132.3 132.6 129.1
125.0 132.3 136.9 137.9 125.9 131.6
136.7 134.5 134.1 135.4 136.0 137.6
130.7 109.9 131.9 115.6 131.3 132.6
2. Supposing that two chemists (A and B) in item #1 above, used two different apparatus. In
order to determine if the precision of these apparatus is significantly different, apply the F-test.
3. Return to the data of chemists A and C in item #1 above. If the true value is 131.0 µg/L.
calculate and compare the respective values of parameter t for these chemists. Can the presence
of a systematic error be concluded for the data of either chemist A or chemist C?
4. The results of experimental measurements on %SO3 in a sample repeated five times were the
following:
24.24, 24.36, 24.97, 24.20, 24.10
Verify whether the third value, which seems to be high compared to the others, should be
considered as an outlier. Use the Grubbs Test at 95% confidence level.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 6 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning