1. What is the conjugate base of the formic acid HCO,H? 2. You have a 5.43 x 10 M solution of HNO, at 25 °C. Calculate the [OH] of this solution? 3. A solution that has a pH of 4.5 is (acidic, basic, neutral)? 4. A solution has a hydronium ion concentration of 3.2 x 10 M. Calculate the pOH. Given the following two equilibria: NICO3 (s) S Ni²+(aq) + CO3 (aq) HCO3 (aq) + H20(E) S CO3² (aq) + H3O*(aq) K1 = 6.6 x 10-9 K2 = 4.8 × 1011 calculate the equilibrium constant for the following reaction: NICO;(s) + H;O (aq) S Ni"(aq) + HCO; (aq) + H,0(t) K3 = ? 2. Heating ammonium hydrogen sulfide leads to decomposition. heat + NH,HS(s) NH3(g) + H,S(g) Predict the effect on the equilibrium of raising the temperature (no change, shift right, shift left). 3. You have two reactions: A - B 20C C5 A +%B
1. What is the conjugate base of the formic acid HCO,H? 2. You have a 5.43 x 10 M solution of HNO, at 25 °C. Calculate the [OH] of this solution? 3. A solution that has a pH of 4.5 is (acidic, basic, neutral)? 4. A solution has a hydronium ion concentration of 3.2 x 10 M. Calculate the pOH. Given the following two equilibria: NICO3 (s) S Ni²+(aq) + CO3 (aq) HCO3 (aq) + H20(E) S CO3² (aq) + H3O*(aq) K1 = 6.6 x 10-9 K2 = 4.8 × 1011 calculate the equilibrium constant for the following reaction: NICO;(s) + H;O (aq) S Ni"(aq) + HCO; (aq) + H,0(t) K3 = ? 2. Heating ammonium hydrogen sulfide leads to decomposition. heat + NH,HS(s) NH3(g) + H,S(g) Predict the effect on the equilibrium of raising the temperature (no change, shift right, shift left). 3. You have two reactions: A - B 20C C5 A +%B
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter4: Stoichiometry: Quantitative Information About Chemical Reactions
Section: Chapter Questions
Problem 109GQ: (a) What is the pH of a 0.105 M HCl solution? (b) What is the hydronium ion concentration in a...
Related questions
Question
![1. What is the conjugate base of the formic acid HCO,H?
2. You have a 5.43 x 10 M solution of HNO, at 25 °C. Calculate the [OH] of this solution?
3. A solution that has a pH of 4.5 is (acidic, basic, neutral)?
4. A solution has a hydronium ion concentration of 3.2 x 10 M. Calculate the pOH.](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F55159ed9-ded2-479d-a538-24c1c0727a51%2Ffa29818c-6f65-4ab3-a25a-ff70df3e630c%2Fqygqbha.jpeg&w=3840&q=75)
Transcribed Image Text:1. What is the conjugate base of the formic acid HCO,H?
2. You have a 5.43 x 10 M solution of HNO, at 25 °C. Calculate the [OH] of this solution?
3. A solution that has a pH of 4.5 is (acidic, basic, neutral)?
4. A solution has a hydronium ion concentration of 3.2 x 10 M. Calculate the pOH.
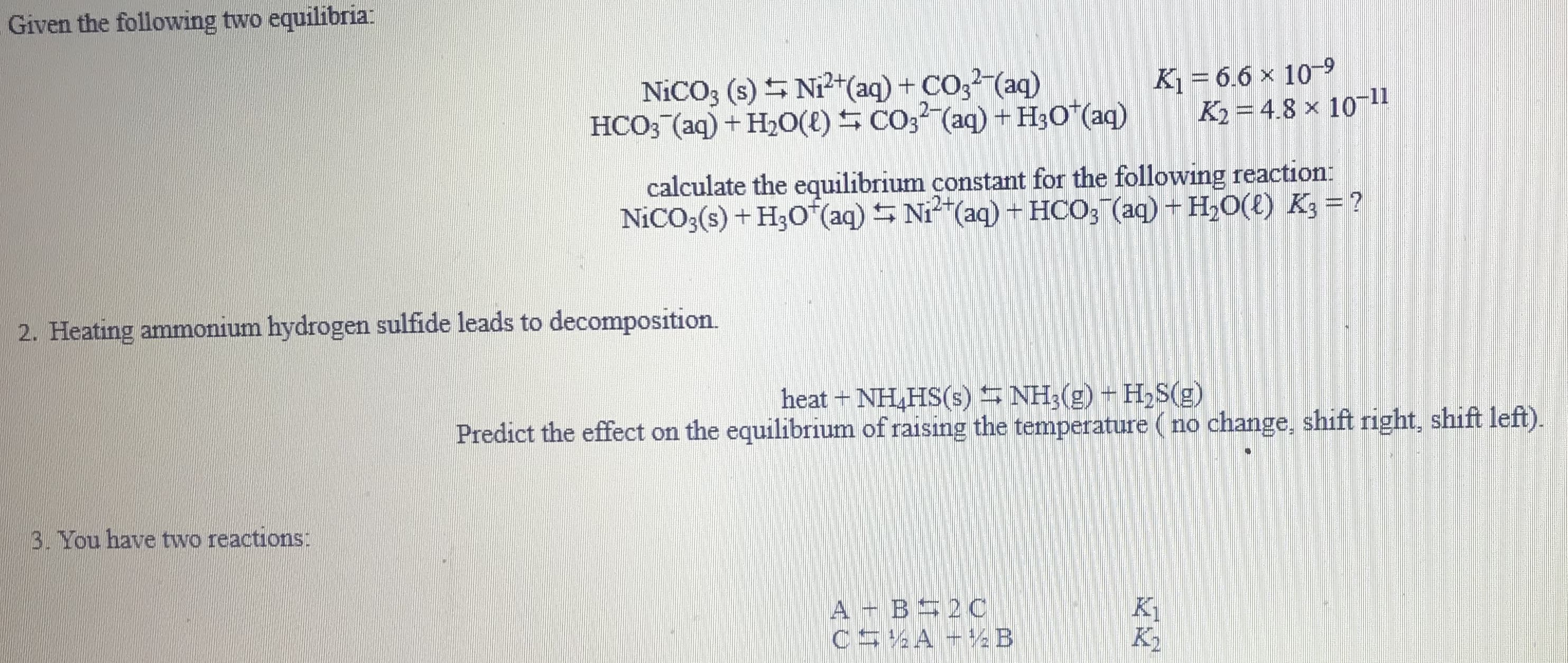
Transcribed Image Text:Given the following two equilibria:
NICO3 (s) S Ni²+(aq) + CO3 (aq)
HCO3 (aq) + H20(E) S CO3² (aq) + H3O*(aq)
K1 = 6.6 x 10-9
K2 = 4.8 × 1011
calculate the equilibrium constant for the following reaction:
NICO;(s) + H;O (aq) S Ni"(aq) + HCO; (aq) + H,0(t) K3 = ?
2. Heating ammonium hydrogen sulfide leads to decomposition.
heat + NH,HS(s) NH3(g) + H,S(g)
Predict the effect on the equilibrium of raising the temperature (no change, shift right, shift left).
3. You have two reactions:
A - B 20C
C5 A +%B
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning