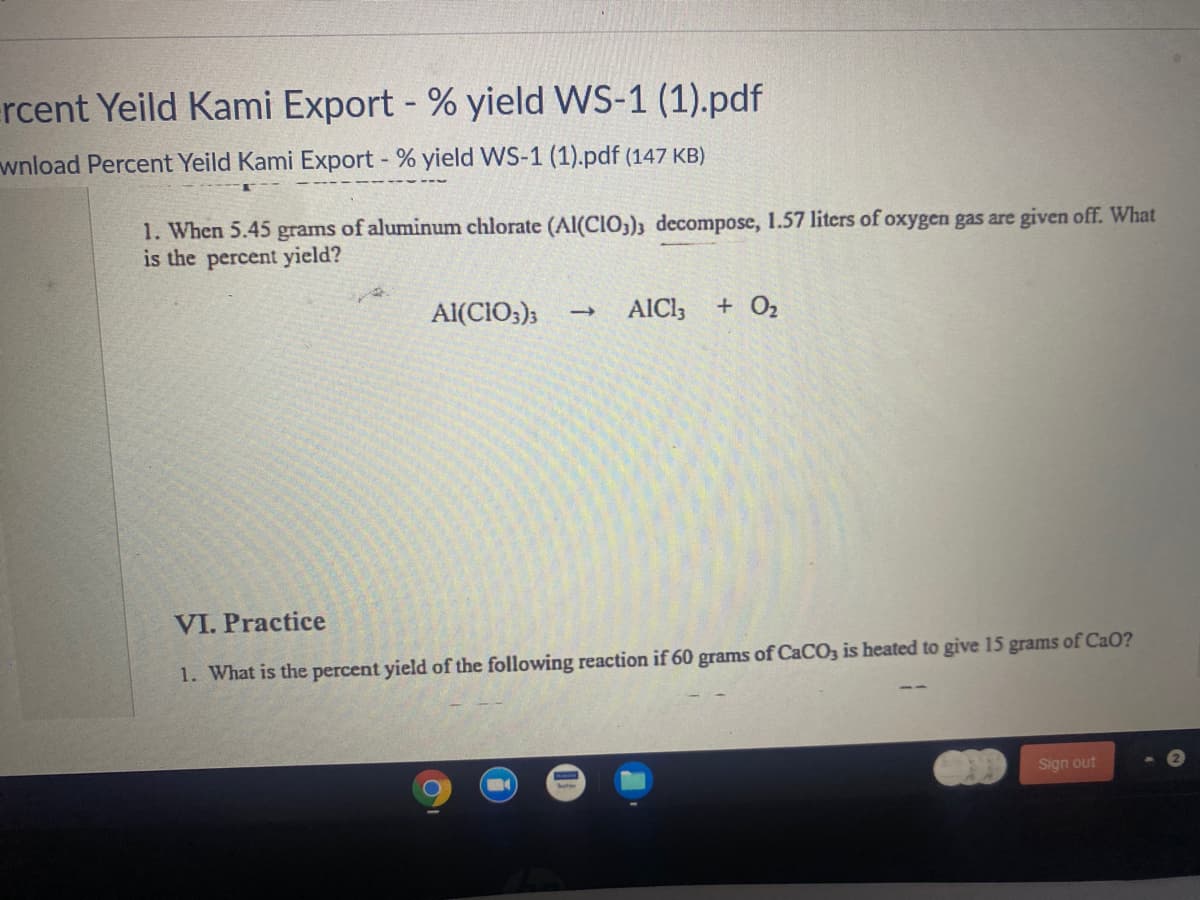
1. When 5.45 grams of aluminum chlorate (Al(CIO;); decompose, 1.57 liters of oxygen gas are given off. What is the percent yield? Al(CIO;); AICI; + O2
Q: What coefficients would you need to balance the following equation, just put the coefficients in the…
A: • The given unbalanced chemical equation is, Mg + HCl → MgCl2 + H2
Q: After mixing these solutions... determine which compound, (NazCO3 or Ni(NO3)2) will be the limiting…
A: Calculation of number of moles of the two reactants given is shown below.
Q: 3. How many grams of oxygen are used in the combustion of 1.9 moles of CH,?
A: Combustion reaction is the reaction between organic compound with Oxygen in the presence of heat to…
Q: A precipitation reaction occurs when 751 mL of 0.825 M Pb(NO,), reacts with 333 mL of 0.916 M KI, as…
A: Given:
Q: Identify 2 things that could happen during an experiment to cause tthe actual yeild to be lower than…
A: Theoretical yield is the maximum amount of product which can be formed in a reaction. Actual yield…
Q: 3.128 Heating 2.40 g of the oxide of metal X (molar mass ofX = 55.9 g/mol) in carbon monoxide (CO)…
A: The moles of X produced is, number of moles=given massmolar mass…
Q: A typical propane, C3H8, tank for an outdoor grill contains 15 pounds of propane. What mass of CO2…
A: Hydrocarbons undergo combustion resulting into production of carbon dioxide and water. They are used…
Q: In a car engine, gasoline (represented by C₈H₁₈) does notburn completely, and some CO, a toxic…
A: (a)
Q: In a combination reaction, 2.22 g of magnesium is heated with 3.75 g of nitrogen. a) Which reactant…
A: in balanced chemical equation number of atoms of each species are equal on both reactent and product…
Q: 3.77 Fermentation is a complex chemical process ofwinemaking in which glucose is converted…
A: According to the mole concept, in terms of mass, the amount of substance in moles is equal to the…
Q: 4. What mass of AgI can be produced from a 0.512-g sample that assays 20.1% AlI,?
A: Balanced equation could be written as All3 + 3Ag+ --> 3 Agl + Al3+ As shown in the equation, one…
Q: Methanol (CH3OH) is a liquid at room temperature with a density of 7.91 x102 kg/m³. In a certain…
A: Percent yield = (Practical yield/theoretical yield)×100
Q: When the equation _Sio2 +_C - _ Sic +. CO - is correctly balanced using whole-number coefficients,…
A: Given compound balance the reaction Here Sio2 have 1 coefficient Carbon have 3 SiC have 1 CO have 2…
Q: A sample of 42.9 g MnO, is added to a solution containing 41.3 g HCl. What is the limiting reactant?…
A: number of moles = mass/molar mass mass = moles × molar mass percent yield ={actual…
Q: A piece of aluminum foil 1.00cm^2 and 0.550-mm thick is allowed to react with bromine to form…
A: Given: The thickness of Al foil=0.550 mm 1 cm =10 mm 0.550 mm ×1 cm10 mm =0.055 cm Volume =1.00 cm2…
Q: Acrylonitrile is used in the production of syntheticfibers, plastics, and rubber goods. It can be…
A: The number of mole of a substance is calculated by dividing the mass (m) of the substance by its…
Q: Mercury (Hg) is typically obtained from the ore Cinnabar, mercury (II) sulfide (HgS), according to…
A: Given that : Mercury (Hg) is typically obtained from the ore Cinnabar, mercury (II) sulfide…
Q: A sample weighing 3.056 g is a mixture of Fe, O, (molar mass = 159.69 g/mol) and Al, 0, (molar mass…
A:
Q: Is each of the following statements true or false? Correctany that are false:(a) A mole of one…
A: Since we only answer up to 3 sub-parts, we’ll answer the first 3. Please resubmit the question and…
Q: Methanol (CH3OH) is a liquid at room temperature with a density of 7.91 *10² kg/m³. In a certain…
A: Methanol has a variety of uses as a fuel, laboratory reagent, solvent, etc. In the given reaction,…
Q: Methanol (CH3OH) is a liquid at room temperature with a density of 7.91 x102 kg/m³. In a certain…
A: In a certain experiment, methanol is made to react with oxygen and carbon dioxide is obtained. For…
Q: .To determine the number of moles of the water of crystallization (n) of a hydrated salt, a student…
A: One possible mistake which usually occurs in gravimetric analysis of determination of loss of water…
Q: 7. When 82.0 g of Ca(CIO,), reacts with Na,SO, in a double displacement reaction, 19.00 g NaCIO, is…
A:
Q: Sulfanilic acid used 25g, Sodium carbonate used 15.2g, Sodium nitrate used 9.94g, 6m HCI used 72ml,…
A: Interpretation - To determine the percentege yield of methyl orange when Sulfanilic acid used 25g,…
Q: elow is a balanced equation of a combustion reaction: C;Hg + 502 - 3CO2 + 4H20 1 mol H,0 4 mol C,H,…
A: Mole ratio is the ratio is the ratio between the amounts in moles of any two compounds involved in a…
Q: by the reaction of hydrochloric acid with manganese(IV) oxide. HCl(aq) + MnO,(s) - MnCl, (aq) + 2…
A: 4HCl (aq) + MnO2(s) ----> MnCl2(aq) + 2H2O (l) + Cl2(g) Mass of HCl = 44.1 g Mass of MnO2 = 43.7…
Q: Methanol (CH3OH) is a liquid at room temperature with a density of 7.91 x102 kg/m³. In a certain…
A: The type of chemical reaction that occurs between methanol and oxygen is a combustion reaction in…
Q: In a combination reaction, 2.22 g of magnesium is heatedwith 3.75 g of nitrogen. (a) Which reactant…
A: Given : Mass of magnesium taken = 2.22 g And mass of nitrogen taken = 3.75 g Atomic mass of…
Q: Methanol (CH3OH) is a liquid at room temperature with a density of 7.91 ×10² kg/m³. In a certain…
A: (a) It is a combustion reaction. (b) Micropipette can be used to measure the volume of methanol.
Q: How many grams of fluorine (F,) are required to produce 264.0 g of CoF,?
A: Since you have posted multiple questions, we are entitled to answer the first only. Please repost…
Q: Hello, these questions were not answered and I was wondering if I could get some help answering…
A: Hey since you have posted a sub-part question we would answer first three sub-parts only. If you…
Q: B. The neutralizing reaction between hydrochloric acid and calcium hydroxide produces calcium…
A: Given Mass of Ca(OH)2 = 0.65 gram Molar mass of Ca(OH)2 = 40 + ( 2×17 ) gm/mole…
Q: In the preparation of Alum, we used (3 mole) reacted with (25 ml) of (1 M) KOH solution and the…
A: Given that - Moles of aluminum, Al = 3 mole Volume of KOH used K=39, Al-27, S-32, H=1= 25 mL…
Q: A sample weighing 3.056 g is a mixture of Fe, O, (molar mass = 159.69 g/mol) and Al, O, (molar mass…
A:
Q: In the reaction, A + B→C + D, if element B is in excess, then: O both A & B are limiting reactants.…
A: A+B→C+Dit means 1 mole of A require 1mole of B
Q: c) Acetylene, C,H, is the fuel used for welding. i) Write the skeleton equation for the combustion…
A: 1) C2H2(g) + O2(g) =CO2(g) +H2O(g) 2)C2H2(g) + 5/2O2(g) =2uCO2(g) +H2O(g)
Q: Many home barbeques are fueled with propane gas (C3H8). What mass of carbon dioxide (in kg) is…
A: The given data contains, volume of propane = 18.9 L =18900 ML density of propane = 0.621 g/ml.…
Q: Given Raw Materials: 500 g calcium polysulphide and 1.5 kg hydrochloric acid Actual Yield: 343.4g…
A: The balanced chemical reaction is : CaS5+2HCl => 4S + CaCl2 + H2S Calculate the moles of calcium…
Q: 10 g of a concentrated HCl solution (density=1.17 g/mL, richness =397.5 g/L) were taken and diluted…
A: Yield of the reaction : The amount of product formed by the calculation of balanced equation and…
Q: The principal component of mothballs is naphthalene, a compound with a molecular mass of about 130…
A: Record the given information,
Q: Which of the following statement(s) is/are false? I) The process of determining the composition of…
A: By chemical analysis, we can detect various ions. By several chemical tests, we can determine which…
Q: Calculate the mass, in grams, of aluminum bromide t 0.154 M aquequs s our olution of the
A:
Q: B. The neutralizing reaction between hydrochloric acid and calcium hydroxide produces calcium…
A:
Q: Determine the limiting reagent and theoretical yield of the product if one starts with 5.0 grams of…
A:
Q: Chlorine gas can be prepared in the laboratory by the reaction of hydrochloric acid with…
A:
Q: 5. A 0.8102-g sample of impure Al,(CO,), decomposed with HCI; the liberated CO, was collected on…
A: Given, Mass of sample = 0.8102g
Q: If 6.58 mol of C3H1, reacts with excess O,, how many moles of CO, will be produced by the following…
A: The given data contains, Moles of C5H12 = 6.58 moles.
Q: Methanol (CH3OH) is a liquid at room temperature with a density of 7.91 x102 kg/m. In a certain…
A: Calculation of mass of methanol: M=V×d=2.91 mL×7.91×102×0.001 g/mL=2.302 g a) The reaction taking…
Q: onsider the reaction of 30.0 mL of 244 M Bal, with 20.0 mL of 0.315 Na3PO4. Bal2(aq) + 2 NazPO4(aq)…
A:
Q: Iron metal is reacted with hydrochloric acid to yield iron(II) chloride and hydrogen gas. A student…
A: Percent yiel id defined as the percent ratio of the actual yield(experimental yield) of the reaction…
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

- Souring of wine occurs when ethanol is converted to acetic acid by oxygen: C2H5OH(l) +O2(g)--->CH3COOH(g)+H2O(l). A 1.00 L bottle of wine labeled as 7.00% (%v/v) ethanol, is found to have a defective seal. Analysis of 1.00 mL showed that there were 0.0274 g acetic acid in that 1.00 mL. The density of ethanol is 0.816 g/mL. (a) What mass of oxygen must leaked into the bottle in pounds(lb) (b) What is the percent yield for the conversion of ethanol to acetic acid, if oxygen is in excess7. Consider a1Msolution ofNa3AsO4. Write the charge and mass balance equations for this system. (please type answer not write by hend)A weight of 0.50 g was taken impure container containing sodium carbonate and bicarbonate. Dissolved in water and then crushed with hydrochloric acid (0.1 N), the burette reading game was at the endpoint of phenolphthalein of 10.5 ml and at the end point of the orange methylation point 30.1 ml. The percentage of sodium carbonate was in ................. knowing that the weights are: Na: 23, C: 12, O: 16
- Use your balanced chemical reaction from the above problem. How many kilograms of the barium product can be produced at 100% yield from the double replacement reaction, made in industry at a larger scale of 675.00 pounds of barium nitrate that is then dissolved in water and mixed with an excess of the potassium sulfate solution. The product is collected by gravimetric filtration and dried. Use 453.592 g = 1 lb. Ba(NO3)2 + K2SO4 ---> BaSO4 + 2KNO3A chemist dissolved 0.08346g of SrCl2 (FW 158.53) and treated it with excess AgNO3 (FW 169.87) to precipitate 0.143g of AgCl (FW 143.32). In his time, the atomic mass of Ag was known to be 107.8 and that of Cl was 35.4 From these values, find the atoimic mass of Sr that the chemist would have calculated.Give typed full explanation After heating 1.1929g CaCO3 and MgCO3 for some minutes the mass of the mixture decreased to 0.8015. The %comp of CaCO3 turned out to be 54.29% and MgCO3 %comp is 45.71%. Calculate the percent error/percent yield.
- In the synthesis of benzoic acid, 3.5 mL of toluene were used and mixed with potassium permanganate solution. In making the potassium permanganate solution, 7 grams of the powder were dissolved in 150 mL of water. The resulting crystals were purified and the yield 1.53 grams. Identify the limiting reagent and compute for the number of moles that it consumed. What is the theoretical yield? What is the percentage yield? MW toluene = 94.14, density=0.87 g/mL , MW KMnO4 = 158, density = 2.7 g/mL , MW Benzoic acid = 122, density = 1.27 g/mLA (3.6500 g) of impure ammonium aluminium sulfate (NH4Al(SO4)2)) was treated with ammonia (NH3(aq)) producing hydrous alumina Al2O3.xH2O. The collected precipitate was filtered, washed, and ignited at 1000 °C to give 0.4935 g Al2O3 (Mw 101.9635 g/mol). Determine: a. % Al2O3 b. %Al c. Express concentration of Al in ppm.Mo in a 0.2711g sample was precipitated giving 1.1682g of (NH4)2PO4.12 MoO3. Find the percentage Mo , P (at wt = 30.97), N=14,Mo=95.9,H=1,O=16
- Use the following atomic masses (in g/mol):Mg = 24.31; O = 16; Ca = 40.08; C = 12.01; Na = 23; H = 1; N = 14.01; S = 32.06; Cl = 35.45; 2.) Limestone consists chiefly of mineral calcite (CaCO3). The carbonate content of 0.5413g of powdered limestone was measured by suspending the powder in water, adding 10mL of 1.392M HCl and heating to dissolve the solid and expel CO2. The excess acid required 39.96mL of 0.1004M NaOH for complete titration to a phenolphthalein end point. Find the % wt of calcite in limestone.An impure sample of Na3PO3 weighing 0.1 g is dissolved in 35 mL of water. A solution containing 45 mL of 3% w/v HgCl2, 30 mL of 10% w/v sodium acetate, and 10 mL of glacial acetic acid is then prepared. After digesting, filtering, and rinsing the precipitate, 0.2857 g of Hg2Cl2 is obtained. Report the purity of the original sample as % w/w Na3PO3.The water in Fell city also contains 30 μg/L of chloroform (formed in the water treatment plant), 5 μg/L of chlorpyriphos (a persticide), and 10 μg/L of chromium VI. Given the reference doses below, calculate the total hazard quotient. State which compound is the total HQ is acceptable. References doses (all mg/kg.d): 0.01 for chloroform, 0.0003 for chlorpyiphos, and 0.003 for chromium VI. Answer: 0.66

