Q: What is the pH of a solution that contains 0.1 M benzoic acid and 0.4 M potassium benzoate? The Ka…
A: Since on dissolving in water benzoic acid give below reaction benzoic acid -------> benzoate ion…
Q: Calculate the pH of 0.50 M sodium acetate, CH3COONa. Ka(CH3COOH) = 1.8 x 10-5 5.72 11.48 2.52…
A: Given;
Q: Determine the pH of a solution that is prepared by dissolving 4.6 g of Ba(OH)2 in 3.3 L water at…
A: Number of moles (n) of solute present in 1 litre solution is called " Molairty (M) " M = n / V (in…
Q: 1. Consider a solution of NaHA for which F = 0.050 mol L¹, K₁1 = 4.70 x 10³ and K₁2 = 1.80 x 10-10,…
A:
Q: What is the pH of a solution that contains 0.15 M benzoic acid and 0.45 M potassium benzoate? The Ka…
A: Assuming benzoic acid dissolves in water and produces y concentration of ions Since the reaction is…
Q: Calculate the pH of the resulting solution when 30.0 mL of 0.16 M KOH are added to 46.0 mL of 0.15 M…
A: When KOH ( a strong base ) is added to HClO ( a weak acid , they would react to form a buffer…
Q: What is the pH of a system that is 0.40 M in NH3 and 0.80 M in NH4Cl? (Kb = 1.8 x10-5 )
A:
Q: Calculate the pH of the solution that results when 16.0 mL of 0.46 M nitric acid (strong acid),…
A:
Q: Find the pH of a 0.15 M solution of ammonia, NH3. Kb = 1.8 X 10-5
A:
Q: Calculate the pH at 25°C of a 0.0099 M solution of a weak base with a Kp of 8.4 x 10⁹. pH
A: pH measures the acidic and basic strength. It has a range of up to 14. The substances having a pH…
Q: What is the pH of a solution that is 0.048 M in HA and also 0.0042 M in NaA? (Ka = 4.1 %3D x 10-6)
A:
Q: What is the pH of a solution that is 0.100 M NH3 and 0.350 M NH4CI? Kb of NH3 = 1.8 x 10-5 9.26 9.80…
A:
Q: What is the pH of a 0.860 M solution of Ca(NO2)2 (Ka of HNO2 is 4.5 x 10-4)?
A: A numerical problem based on pH concept, which is to be accomplished.
Q: Calculate the pH of a solution that is 0.410 M in HOCI and 0.050 M in NaOCI. [Ka(HOCI) = 3.2 x 10-8)…
A: Concentration of HOCl=0.410M Concentration of NaOCl=0.050M Ka(HOCl)=3.2 × 10^-8
Q: A 1.00 L solution contains propanoic acid (C3 H5 O2 H) with a concentration of 0.350 mol/L, and…
A:
Q: Calculate the pH of a 100.0 mL solution containing 9.06 g KClO
A:
Q: 6.5 g of sodium acetate was dissolved in 100 mL of water to give a final volume of 106 mL. If the Ka…
A: Sodium acetate dissociates in water into sodium and acetate ions. Sodium ions react very little with…
Q: The PH of 0.0296M HA ( Ka 8.4 *10-9) plus * . . 0.1026 M NaA is 4.3 2 O 8.6 7.5 O
A: The question is based on the concept of PH calculations. we have been given a weak acid and its…
Q: what is the ph of a 0.85 M solution of pyridine (C5H5N, Kb = 1.70 x 10 ^-9)
A: Pyridine is a weak base. pOH of a weak base. pOH = -log [OH-] pH = 14 - pOH For a weak base like…
Q: What is the pH of a solution when 25 ml, 0.200 M of salicylic acid CeH:(OH)COOH is diluted to 100.0…
A: Given: Concentration of salicylic acid i.e. HA (Assuming it HA as its monoprotic acid) = 0.200 M…
Q: Calculate the pH in the solution formed by adding 10.0 mL of 0.050 M NaOH to 40.0 mL of 0.0250 M…
A: The number of moles of benzoic acid and NaOH in the solution can be calculated using equation (1),
Q: What is the pH of a 0.830 M solution of Ca(NO₂)₂ (Ka of HNO₂ is 4.5 × 10⁻⁴)?
A: The chemical equation may be written as: The given data is: Concentration of Ca(NO2)2 =0.830M Ka of…
Q: A titration of 0.100 M NaOH is titrated into 25.0 ml of a 0.100 M HCHO2 solution (Ka= 1.80 x 10^-4)…
A: Acid-base titration: It is an analytical method used for determining the concentration of unknown…
Q: Calculate the pH of the solution resulting from the addition of 20.0 ml. of 0.100 M NaOH to 30.0 mL…
A: pH is the measure of acidity. More acidic the solution, less will be the value of pH. pH = -log…
Q: Calculate the pH of a solution made by adding 0.123 g of solid NaOH to 275 mL of 0.350 M HNO2 (Ka =…
A:
Q: A 45.0 ml sample of 0.250 M CH3CH2NH2 was mixed with 32.0 mL of 0.225 M HNO3. Kp = 5.6 x 104 %3D а.…
A: mmol of ethylamine = 45*0.250 = 11.25 = 11.25*10^-3 mol mmol of HNO3 = 7.2 = 7.2*10^-3 mol…
Q: Which of the following solutions will have the lowest pH value 2.0 M CH3COOH (Ka = 1.8 x 10-5) %3D…
A: The concentration of hydrogen ion for acetic acid can be calculated as follows-
Q: Determine the pH of a 0.15 M solution of Ca(BrO2)2 at 25 oC. At the same temperature, Ka for HBrO2…
A: Answer: Conjugate acid of weak base and conjugate base of weak acid gets hydrolyzed on adding in…
Q: Calculate the pH of a solution which is 0.50 M in HA (a weak acid) and 0.80M in NaA. (Ka for HA =…
A: Henderson-Hasselbalch equation explains the relationship between pH of solution and pKa of acid for…
Q: Determine the pH at 25C of a solution prepared by dissolving 0.35 mole of ammonium chloride in 1. L…
A: Concentration of ammonia Volume of ammonia solution Number of moles of ammonium chloride added =…
Q: Calculate the pH of the solution formed when 45.0 mL of 0.100 M NaOH is added to 50.0 mL of 0.100 M…
A: NaOH will react completely with acid CH3COOH as its a strong base NaOH + CH3COOH -------->…
Q: Calculate the pH of the solution when 45.0 mL of 0.25 M HNO2 is mixed with 45.0 mL of 0.35 M NaOH.…
A:
Q: What is the pH in the titration of 100 ml of a 0.030 M solution of CH3COOH with 15.00 ml of 0.150 M…
A:
Q: Calculate the pH of a solution which is 0.50 M in HA (a weak acid) and 0.80M in NaA. (Ka for HA =…
A:
Q: Calculate the pH of a 120mL of a solution containing 0.5g of hydrofluoric acid. Ka = 6.6 x 10^-4
A: pH = -log[H+] Molar concentration = number of moles of solute/volume of solution (in L) number of…
Q: Calculate the pH of a solution prepared by dissolving 0.040 moles of sodium nitrite (NaNO2) in 200…
A: When HNO2 and NaNO2 are mixed, they form buffer solution HNO2/NO2-. And the pH of a solution is…
Q: Calculate the pH of a solution of 6.5 x 10⁹ mol of Ca(OH)2 in 10.0 L of water. Ksp(Ca(OH)₂) = 6.5 x…
A:
Q: Calculate the pH of a solution formed by mixing 100.0 mL of 0.100 M NaF and 100.0 mL of 0.040 M HCI.…
A: pH = pKa + log [salt]/[acid] pKa = -log Ka Number of mmols = Molarity x volume (mL)
Q: What would be the pH of a 0.12 M solution of a weak acid with Ka = 7.51 x 10^-5? HA <=>…
A: [HA] <===> H+ +A- I 0.12 0 0 C -x…
Q: Find the pH of a 0.010 M solution of morphine, another weak base with Kb = 1.6 X 10-6
A:
Q: Calculate the pH of 0.65 M of a weak acid HA with Ka=2.56 x 10^-5 a. 5.59 b. 2.89 c. 2.78 d.…
A:
Q: Determine the pH of a solution that is prepared by dissolving 8.7 g of Ba(OH)2 in 2 L water at…
A:
Q: What is the pH of a 0.862 M NH3 solution that has a Kb = 1.8 x 10-5?
A:
Q: What is the pH of a solution that is 0.32 M H(C6H5COO) and 0.12 M Na(C6H5COO)? The Ka of…
A: Answer:- This question is answered by using the simple concept of calculation of pH of solution…
Q: What is the pH in the titration of 100 ml of a 0.030 M solution of CH3COOH with 21.00 ml of 0.150 M…
A: Given that : The volume of CH3COOH = 100 mL The molarity of CH3COOH = 0.030 M The volume of NaOH =…
Q: 3. Ammonia, a weak base, has a Kb value of 1.75 x 10 at 298 K a) Determine the pH of 50.0 mL of 0.1…
A: A) we will use weak base concept for getting pH
Q: Calculate ph of a 0.94 M solution of a weak base with KB= 1.97 x 10^-9
A:
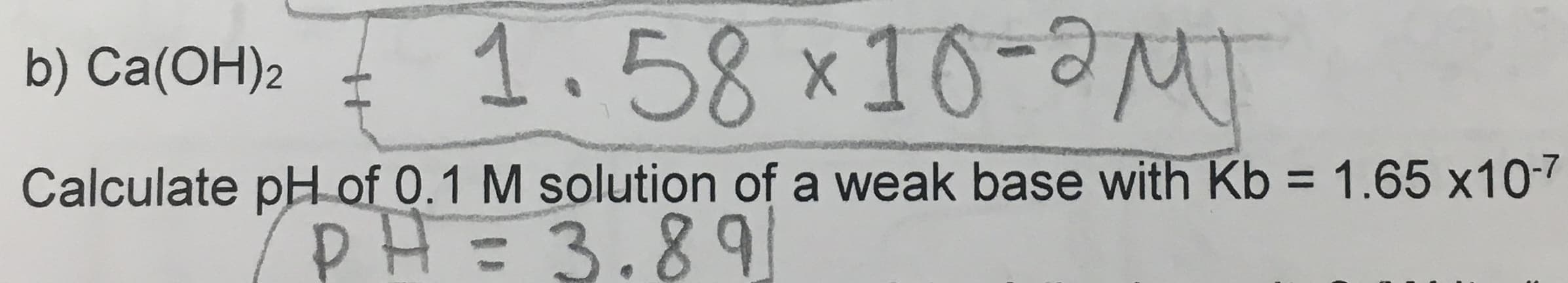
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

- A solution is made by dissolving 0.111 moles of N2H4 (Kb = 3.0 x 10-6 ) and 0.199 moles of N2H5Cl in water and diluting to 1.00 L. What is the pH of the resulting solution?What is the pH value of saturated lime (Ca(OH)2) solution? (Ksp =Kb=8x10 -10)A 0.345 M of generic weak acid was prepared and has a Kb = 3.91 x 10-10. What is the pH of the weak acid?
- What is the pH of the solution which results from mixing 75 mL of 0.50 M NH3(aq) and 75 mL of 0.50 HCl(aq) at 25 ºC? (Kb for NH3 = 1.8 x 10-5)Calculate the pH of the solution that results when 20.0 mL of 0.1750 M formic acid is: [Ka = 1.8 x 10-4] (a) diluted to 45.0 mL with distilled water. (b) mixed with 25.0 mL of 0.140 M NaOH solution. (c) mixed with 25.0 mL of 0.200 M NaOH solution. (d) mixed with 25.0 mL of 0.200 sodium formate solutionWhat is the pH and fraction of association of a 0.100 M solution of a weak base with Kb = 1.00 X 10-5?
- What is the pH of the solution after mixing 0.188 g of Mg(OH)2 (MW=58.321 g/mol) with 18.1 mL of 0.0173 M HCl? The resulting solution was diluted to 100 mL. Round your calculated value for pH to two figures to the right of the decimal point.You have 500 mL of a buffer solution conatining 0.154 M acetic acid (CH3COOH) and 0.158 M sodium acetate (CH3COONa). What will the pH of this solution be after the addition of 20.0 mL of 1.00 M NaOH solution? (Ka = 1.39 x 10^-5)What is the pH in the titration of 100 ml of a 0.030 M solution of NH3 with 21.00 ml of 0.150 M HCl? kb = 1.8 x 10^-5
- A 0.100 M HNO3 solution is used to titrate 50.0 mL of 0.100 M trimethylamine ((CH3)3N, Kb = 7.4 x 10-5). Identify the type of solution indicated for the following points in the titration and calculate the resulting pH. When 50.2 mL of titrant are added.Calculate the pH of a carbonate buffer system that contains 0.0065 M HCO3 and 0.0084 M CO32-. The Ka of HCO3- is 4.7 X 10-11.What is the pH in a solution of 1.0 M H2A (Ka1 = 1.0 10–6; Ka2 = 1.0 10–10) at 25oC?



