10 mL of 1.8 M acetic acid (CH3COOH) is titrated with 1.0 M sodium hydroxide (NaOH). The titration curve is shown. What is the approximate pK₁? pK₂ = 0.5 12 108 fraction converted: 0.5 吉 6 4 2 0 At the pK₁, what fraction of the carboxyl group will have been converted to COO¯? Express your answer as a decimal, not a percent. 0 5 10 15 Volume of base (mL) 20 25
10 mL of 1.8 M acetic acid (CH3COOH) is titrated with 1.0 M sodium hydroxide (NaOH). The titration curve is shown. What is the approximate pK₁? pK₂ = 0.5 12 108 fraction converted: 0.5 吉 6 4 2 0 At the pK₁, what fraction of the carboxyl group will have been converted to COO¯? Express your answer as a decimal, not a percent. 0 5 10 15 Volume of base (mL) 20 25
Chapter2: Decimal Fractions
Section: Chapter Questions
Problem 1RP
Related questions
Question
100%
What is the approximate p?apa?
I've tried entering 0, 1 and 0.5 and got marked wrong for the first part.
The second part is correct.
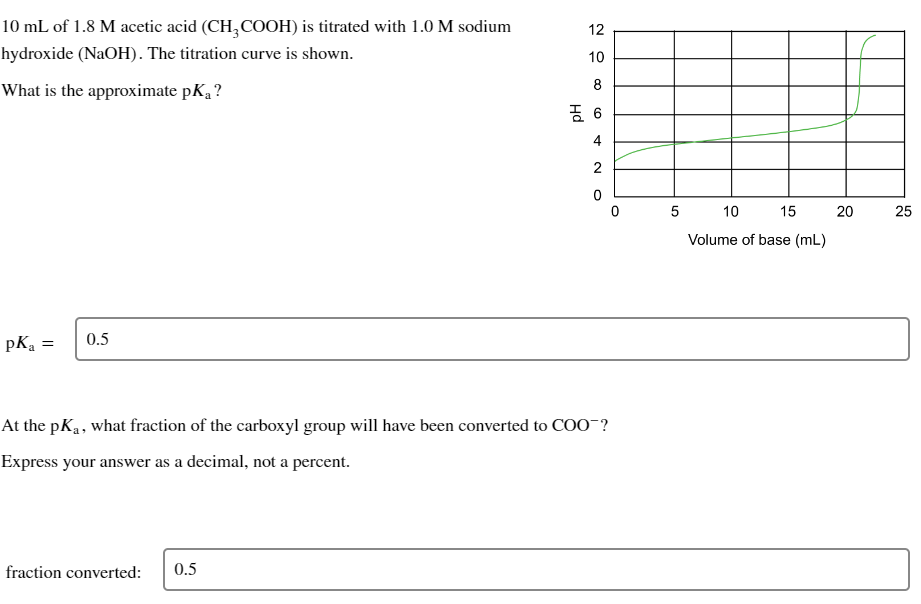
Transcribed Image Text:10 mL of 1.8 M acetic acid (CH3COOH) is titrated with 1.0 M sodium
hydroxide (NaOH). The titration curve is shown.
What is the approximate pK₁?
pKa
=
0.5
fraction converted:
12 10 8
At the pK₁, what fraction of the carboxyl group will have been converted to COO-?
Express your answer as a decimal, not a percent.
0.5
6
4
2
0
0
5
10
15
Volume of base (mL)
20
25
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 3 images

Recommended textbooks for you

