13. A copper block of mass 2.5 k g is heated in a furnace to a temperature of 500 °C and then placed on a large ice block. What is the maximum amount of ice that can melt? (Specific heat of copper =0.39Jg 'K' ; heat of 335 J g''). fusion of water = ||
13. A copper block of mass 2.5 k g is heated in a furnace to a temperature of 500 °C and then placed on a large ice block. What is the maximum amount of ice that can melt? (Specific heat of copper =0.39Jg 'K' ; heat of 335 J g''). fusion of water = ||
Principles of Heat Transfer (Activate Learning with these NEW titles from Engineering!)
8th Edition
ISBN:9781305387102
Author:Kreith, Frank; Manglik, Raj M.
Publisher:Kreith, Frank; Manglik, Raj M.
Chapter1: Basic Modes Of Heat Transfer
Section: Chapter Questions
Problem 1.72P: 1.72 An ice chest (see sketch) is to constructed from styrofoam . If the wall of the chest is 5-cm...
Related questions
Question
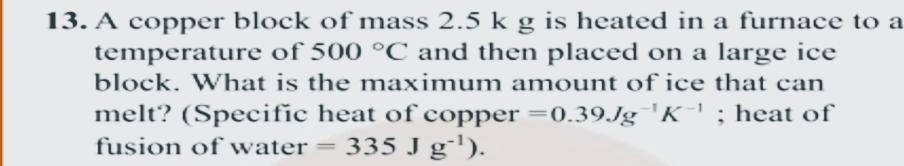
Transcribed Image Text:13. A copper block of mass 2.5 k g is heated in a furnace to a
temperature of 500 °C and then placed on a large ice
block. What is the maximum amount of ice that can
melt? (Specific heat of copper =0.39.Jg 'K' ; heat of
335 J g'').
fusion of water =
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Heat Transfer (Activate Learning wi…
Mechanical Engineering
ISBN:
9781305387102
Author:
Kreith, Frank; Manglik, Raj M.
Publisher:
Cengage Learning

Principles of Heat Transfer (Activate Learning wi…
Mechanical Engineering
ISBN:
9781305387102
Author:
Kreith, Frank; Manglik, Raj M.
Publisher:
Cengage Learning