14.16 Consider the following equilibrium process at 700°C: 2H2 (g) + S2(g)= 2H2S(g) Analysis shows that there are 2.50 moles of H2, 1.35 × 10 mole of S2, and 8.70 moles of H2S present in a 12.0-L flask. Calculate the equilibrium constant K. for the reaction. Pag
14.16 Consider the following equilibrium process at 700°C: 2H2 (g) + S2(g)= 2H2S(g) Analysis shows that there are 2.50 moles of H2, 1.35 × 10 mole of S2, and 8.70 moles of H2S present in a 12.0-L flask. Calculate the equilibrium constant K. for the reaction. Pag
Chemistry & Chemical Reactivity
9th Edition
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter15: Principles Of Chemical Reactivity: Equilibria
Section: Chapter Questions
Problem 7PS: The reaction PCl5(g) PCl3(g) + Cl2(g) was examined at 250 C. At equilibrium, [PCl5] = 4.2 105...
Related questions
Question
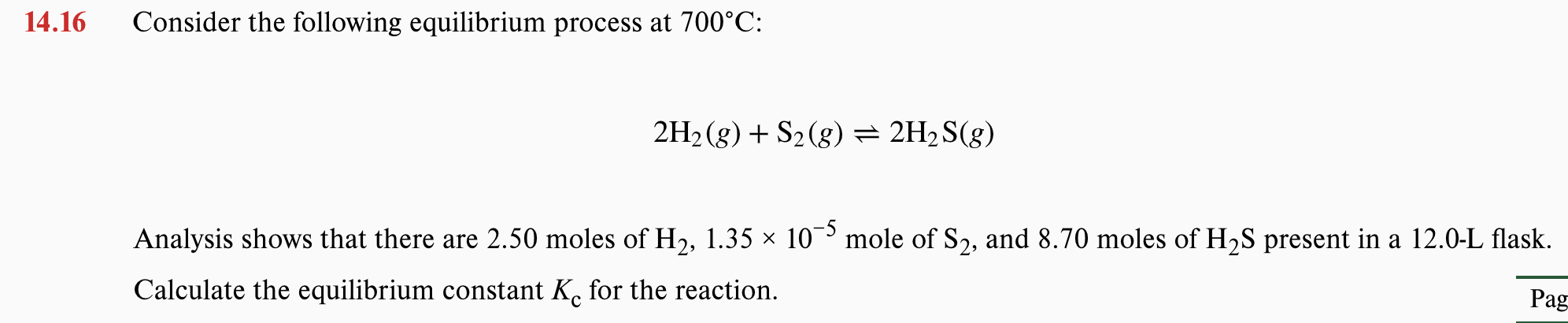
Transcribed Image Text:14.16
Consider the following equilibrium process at 700°C:
2H2 (g) + S2(g)= 2H2S(g)
Analysis shows that there are 2.50 moles of H2, 1.35 × 10 mole of S2, and 8.70 moles of H2S present in a 12.0-L flask.
Calculate the equilibrium constant K. for the reaction.
Pag
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 3 images

Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning