140 a decay 209 83 130 - (4-152) 120 - 184 74 110 - 4- 1.40) 100 Region shown in B 90구 B- decay 80 - 1Ag -1.28) 107 47 70 - 60 50 - 56 =1 40 - 4-1.15) 30 - B* emission and/or е сapture 20 Ne (실-10) 10 - 10 20 30 40 50 60 70 80 90 A Protons (Z) Figure 23.2 A plot of number of neutrons vs. number of protons for the stable nuclides. Neutrons (N) ZIN
140 a decay 209 83 130 - (4-152) 120 - 184 74 110 - 4- 1.40) 100 Region shown in B 90구 B- decay 80 - 1Ag -1.28) 107 47 70 - 60 50 - 56 =1 40 - 4-1.15) 30 - B* emission and/or е сapture 20 Ne (실-10) 10 - 10 20 30 40 50 60 70 80 90 A Protons (Z) Figure 23.2 A plot of number of neutrons vs. number of protons for the stable nuclides. Neutrons (N) ZIN
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter32: Radiochemical Methods
Section: Chapter Questions
Problem 32.2QAP
Related questions
Question
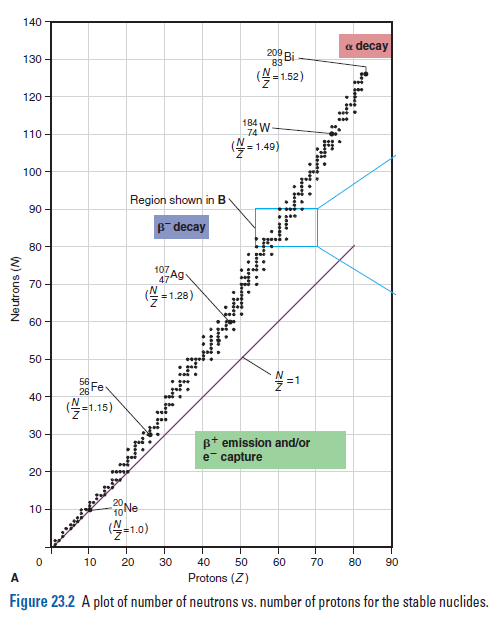
Problem Which of the following nuclides would you predict to be stable and which radioactive: (a) 18/10Ne; (b) 32/16S; (c) 23/690Th; (d) 123/56Ba? Explain.
Plan In order to evaluate the stability of each nuclide, we determine the N and Z values,the N/Z ratio from (A - Z )/Z, the value of Z, stable N/Z ratios (from as shown), and whether Z and N are even or odd.
Transcribed Image Text:140
a decay
209
83
130 -
(4-152)
120 -
184
74
110 -
4- 1.40)
100
Region shown in B
90구
B- decay
80 -
1Ag
-1.28)
107
47
70 -
60
50 -
56
=1
40 -
4-1.15)
30 -
B* emission and/or
е сapture
20
Ne
(실-10)
10 -
10
20
30
40
50
60
70
80
90
A
Protons (Z)
Figure 23.2 A plot of number of neutrons vs. number of protons for the stable nuclides.
Neutrons (N)
ZIN
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning
