15. Predict the total pressure in Container C if the initial pressure in Container A was tripled and Container B was reduced by one-third then mixed in Container C. Container A Oxygen Pressure 287 kPa Solve, showing all calculation work. Container B Nitrogen Pressure 429 kPa Container C Oxygen+ Nitrogen Pressure Ptotal =
15. Predict the total pressure in Container C if the initial pressure in Container A was tripled and Container B was reduced by one-third then mixed in Container C. Container A Oxygen Pressure 287 kPa Solve, showing all calculation work. Container B Nitrogen Pressure 429 kPa Container C Oxygen+ Nitrogen Pressure Ptotal =
Chemistry: Matter and Change
1st Edition
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Chapter13: Gases
Section: Chapter Questions
Problem 96A
Related questions
Question
P total=P1+P2+P3
P container A =3x287kPa
=861 kPa
P container B= 1/3*429kPa
= 143 kPa
P total=861 kPa+143 kPa
= 1004 kPa
Apply the significant figure rule to the final answer.
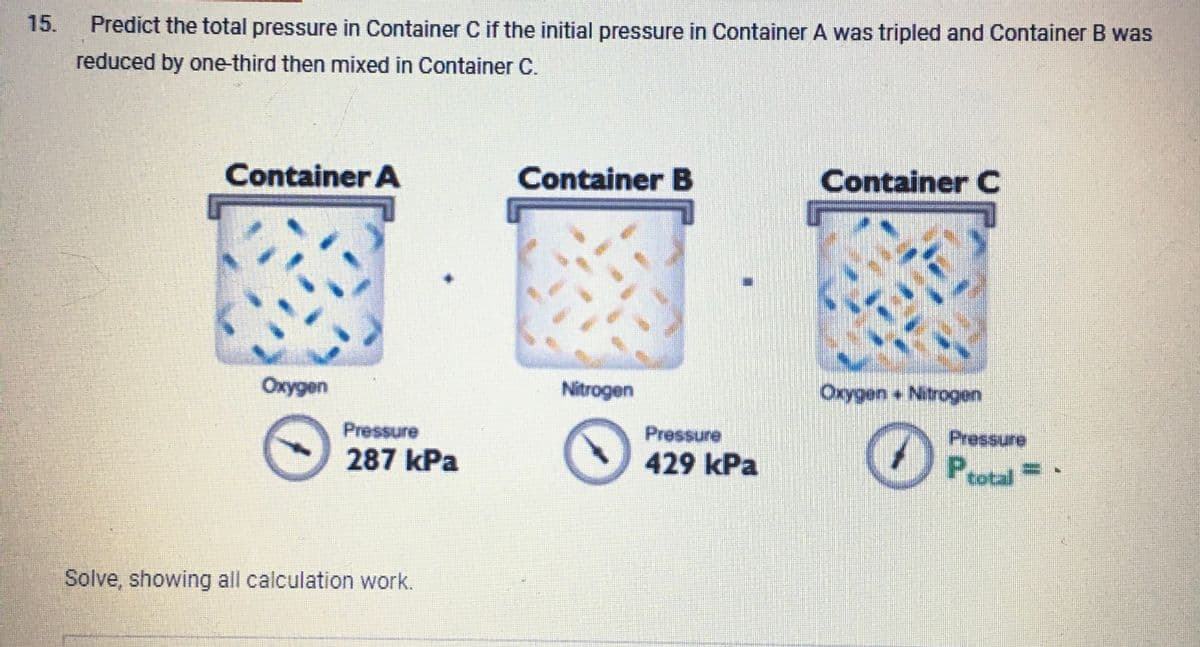
Transcribed Image Text:15.
Predict the total pressure in Container C if the initial pressure in Container A was tripled and Container B was
reduced by one-third then mixed in Container C.
Container A
Oxygen
Pressure
287 kPa
Solve, showing all calculation work.
Container B
Nitrogen
Pressure
429 kPa
Container C
UN
Oxygen + Nitrogen
O
Pressure
Ptotal=
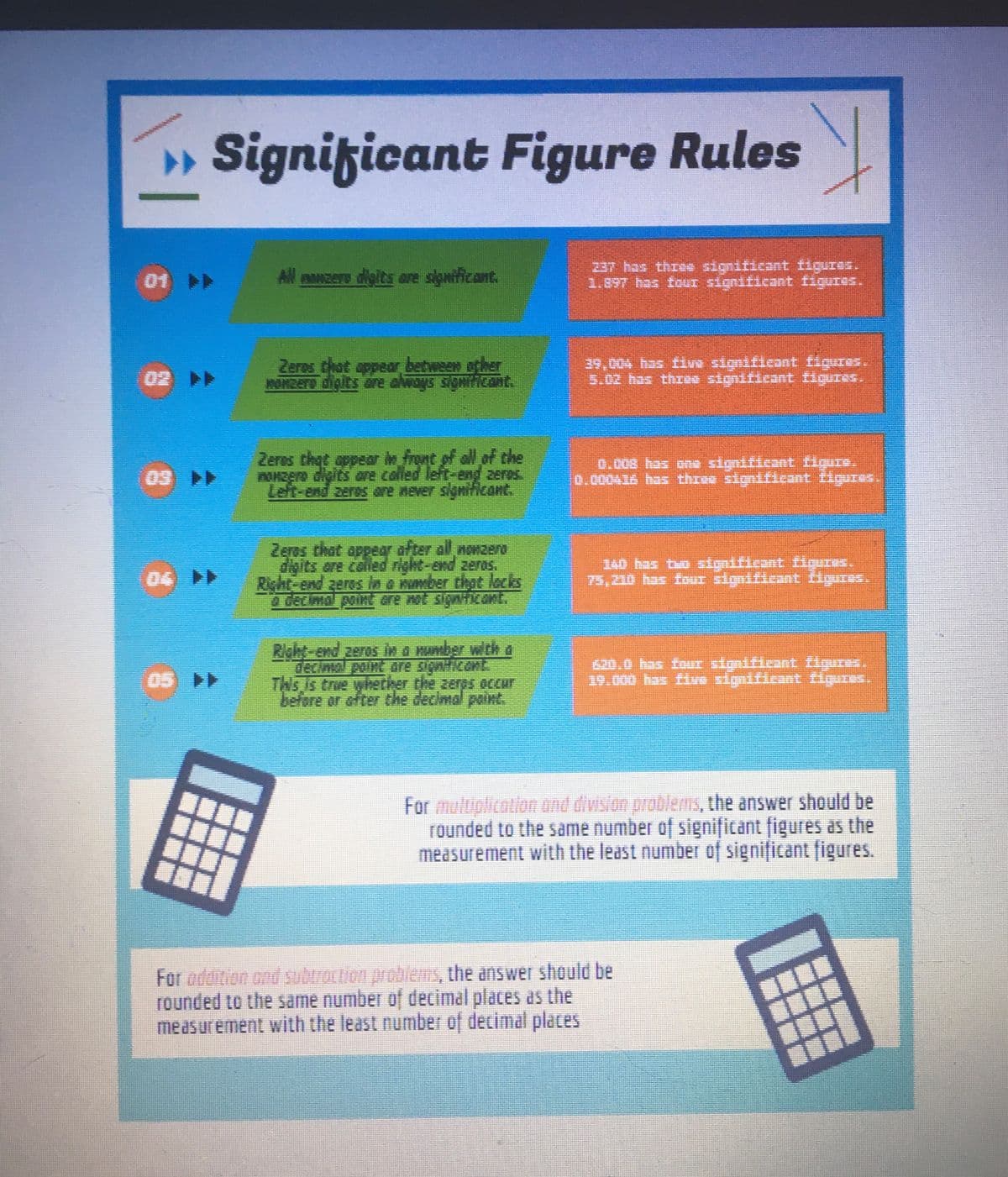
Transcribed Image Text:» Significant Figure Rules
01 >>
All manzere digits are significant.
Zeres that appear between other
HOMZETA digits are always significant.
Zeros that appear in front of all of the
nonzero daits are called left-end zeros.
Left-end zeros are never significant.
Zeros that appear after all nonzero
digits are called right-end zeros.
à decimal point are not significant.
Right-end zeros in a number with a
This is true whether the seres occur
before or after the decimal point.
337 has three significant figures.
1.897 has four significant figures.
39,004 has five significant figures.
5.02 has three significant figures.
0.008 has one significant figure.
0.000416 has three significant figures.
140 has two significant figures.
75,310 has four significant figures.
620.0 has four significant figures.
19.000 has five significant figures.
For multiplication and division problems, the answer should be
rounded to the same number of significant figures as the
measurement with the least number of significant figures.
For addition and subtraction problems, the answer should be
rounded to the same number of decimal places as the
measurement with the least number of decimal places
1
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning