166. 40.0 g of ice cubes at 0.0°C are combined with 150. g of liquid water at 20.0°C in a coffee cup calorimeter. Calculate the final temperature reached, assuming no heat loss or gain from the surroundings. (Data: specific heat capacity of H20(1), c = 4.18 J/g.°C; H2O(s) → H2O(1) A) 0.0 %3D НаО() B) 10.6 AH= 6.02 kJ/mol) C) 30.7 D) 43.2 E) 56.4 %3D
166. 40.0 g of ice cubes at 0.0°C are combined with 150. g of liquid water at 20.0°C in a coffee cup calorimeter. Calculate the final temperature reached, assuming no heat loss or gain from the surroundings. (Data: specific heat capacity of H20(1), c = 4.18 J/g.°C; H2O(s) → H2O(1) A) 0.0 %3D НаО() B) 10.6 AH= 6.02 kJ/mol) C) 30.7 D) 43.2 E) 56.4 %3D
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter8: Thermochemistry
Section: Chapter Questions
Problem 88QAP: A sample of sucrose, C12H22O11, is contaminated by sodium chloride. When the contaminated sample is...
Related questions
Question
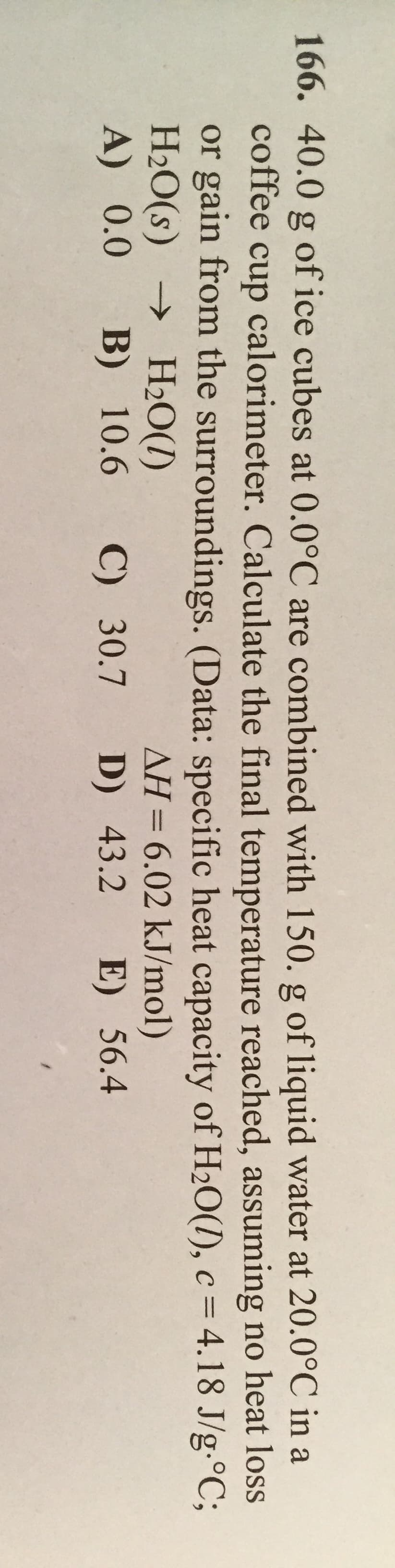
Transcribed Image Text:166. 40.0 g of ice cubes at 0.0°C are combined with 150. g of liquid water at 20.0°C in a
coffee cup calorimeter. Calculate the final temperature reached, assuming no heat loss
or gain from the surroundings. (Data: specific heat capacity of H20(1), c = 4.18 J/g.°C;
H2O(s) → H2O(1)
A) 0.0
%3D
НаО()
B) 10.6
AH= 6.02 kJ/mol)
C) 30.7 D) 43.2 E) 56.4
%3D
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 3 images

Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning