17. At a certain temperature K. = 0.434 for the reaction 2 SO2(8) + 102(g) 2 2 SO3(g). What is the value of K, for the reaction 1 SO3(8) 2 1 SO2(8) + 2 O2(g)? A. K. = 2.30 В. К. - 0.0217 С. К. - 1.15 D. K. = 1.52 18. The reaction 2 SO2(8) + 102(8) 22 SO3(8) is catalyzed by V205, a homogeneous catalyst, and by platinum metal, a heterogeneous catalyst. A similar reaction is catalyzed in our bodies by an enzyme called sulfite oxidase. Which of the following statements concerning catalysts is incorrect? A. Catalysts do not get used up during chemical reactions because they do not actually react, they just bring the reactants together more often and with higher energy. The active site of enzymes like sulfite oxidase lowers the energy of the transition state of the reaction that is being catalyzed. В. C. Catalysts speed up reactions by providing a rate-determining step with a lower activation energy than that of the uncatalyzed reaction. D. Heterogeneous catalysts speed up reactions by absorbing the reactants onto the catalyst surface: this increases the reactivity of the reactants.
17. At a certain temperature K. = 0.434 for the reaction 2 SO2(8) + 102(g) 2 2 SO3(g). What is the value of K, for the reaction 1 SO3(8) 2 1 SO2(8) + 2 O2(g)? A. K. = 2.30 В. К. - 0.0217 С. К. - 1.15 D. K. = 1.52 18. The reaction 2 SO2(8) + 102(8) 22 SO3(8) is catalyzed by V205, a homogeneous catalyst, and by platinum metal, a heterogeneous catalyst. A similar reaction is catalyzed in our bodies by an enzyme called sulfite oxidase. Which of the following statements concerning catalysts is incorrect? A. Catalysts do not get used up during chemical reactions because they do not actually react, they just bring the reactants together more often and with higher energy. The active site of enzymes like sulfite oxidase lowers the energy of the transition state of the reaction that is being catalyzed. В. C. Catalysts speed up reactions by providing a rate-determining step with a lower activation energy than that of the uncatalyzed reaction. D. Heterogeneous catalysts speed up reactions by absorbing the reactants onto the catalyst surface: this increases the reactivity of the reactants.
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter17: Spontaneity, Entropy, And Free Energy
Section: Chapter Questions
Problem 121CP: If wet silver carbonate is dried in a stream of hot air. the air must have a certain concentration...
Related questions
Question
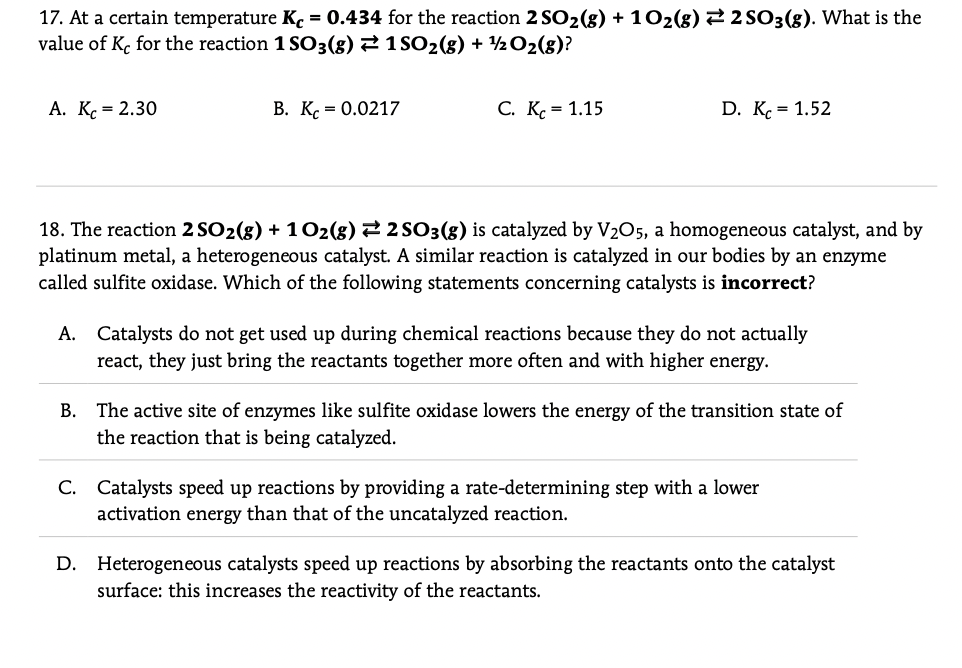
Transcribed Image Text:17. At a certain temperature K. = 0.434 for the reaction 2 SO2(8) + 102(g) 2 2 SO3(g). What is the
value of K, for the reaction 1 SO3(8) 2 1 SO2(8) + 2 O2(g)?
A. K. = 2.30
В. К. - 0.0217
C. K. = 1.15
D. K. = 1.52
18. The reaction 2 SO2(8) + 102(8) 22 SO3(8) is catalyzed by V205, a homogeneous catalyst, and by
platinum metal, a heterogeneous catalyst. A similar reaction is catalyzed in our bodies by an enzyme
called sulfite oxidase. Which of the following statements concerning catalysts is incorrect?
A. Catalysts do not get used up during chemical reactions because they do not actually
react, they just bring the reactants together more often and with higher energy.
The active site of enzymes like sulfite oxidase lowers the energy of the transition state of
the reaction that is being catalyzed.
В.
C. Catalysts speed up reactions by providing a rate-determining step with a lower
activation energy than that of the uncatalyzed reaction.
D. Heterogeneous catalysts speed up reactions by absorbing the reactants onto the catalyst
surface: this increases the reactivity of the reactants.
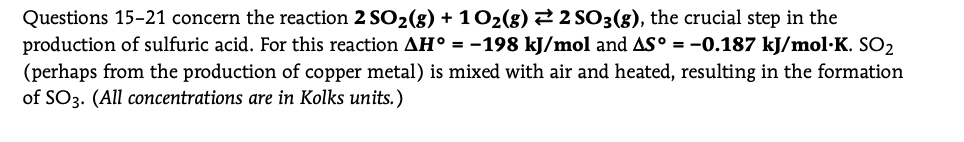
Transcribed Image Text:Questions 15-21 concern the reaction 2 SO2(8) + 102(8) 22 SO3(g), the crucial step in the
production of sulfuric acid. For this reaction AH° = -198 kJ/mol and AS° = -0.187 kJ/mol·K. SO2
(perhaps from the production of copper metal) is mixed with air and heated, resulting in the formation
of SO3. (All concentrations are in Kolks units.)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning