189 mL of a 1.9 M solution of KBr is diluted to 668 mL. What is the new concentration of the solution (in M to 2 decimal places)? Do not input units on the Canvas answer, but they should be visible on your uploaded file.
189 mL of a 1.9 M solution of KBr is diluted to 668 mL. What is the new concentration of the solution (in M to 2 decimal places)? Do not input units on the Canvas answer, but they should be visible on your uploaded file.
Chapter12: The Liquids And Solids Around Us: Especially Water
Section: Chapter Questions
Problem 47E
Related questions
Question
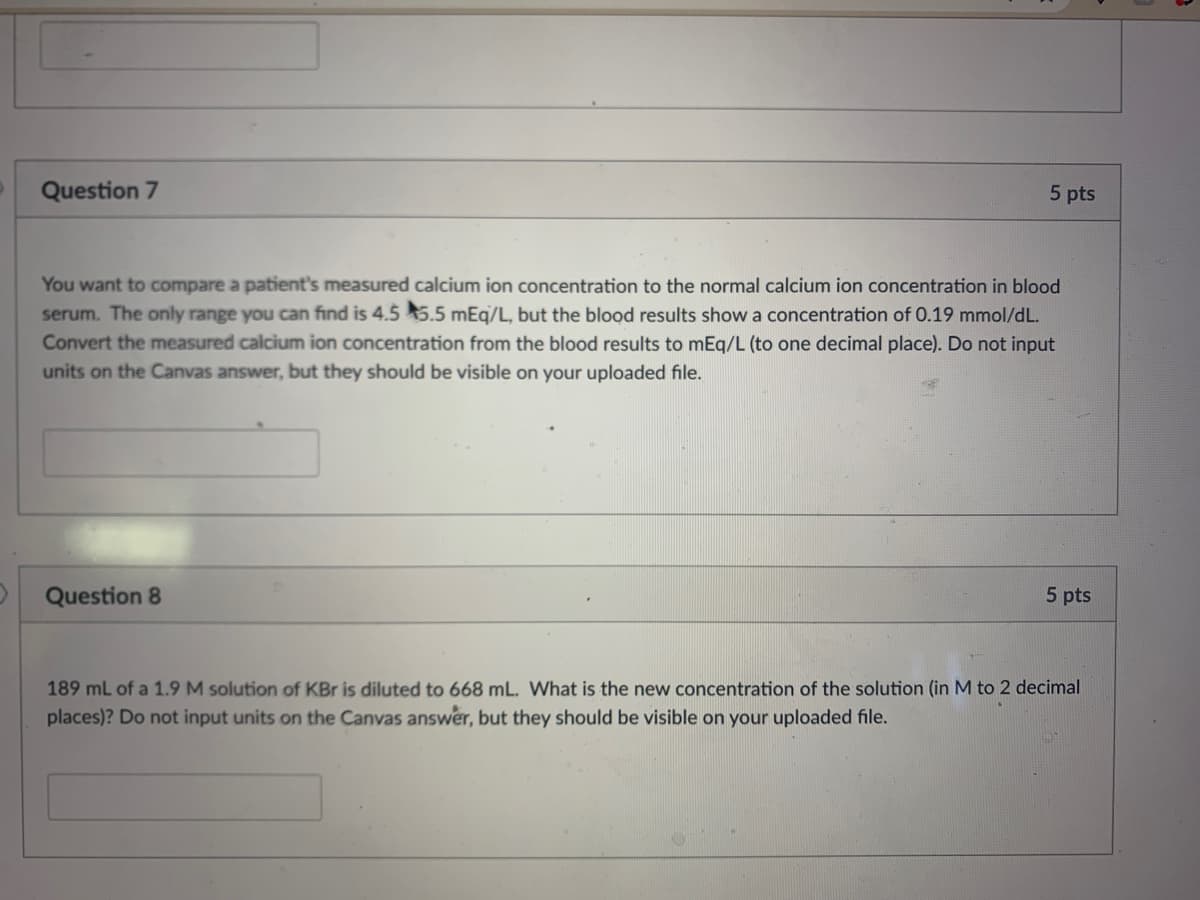
Transcribed Image Text:Question 7
5 pts
You want to compare a patient's measured calcium ion concentration to the normal calcium ion concentration in blood
serum. The only range you can find is 4.5 5.5 mEq/L, but the blood results show a concentration of 0.19 mmol/dL.
Convert the measured calcium ion concentration from the blood results to mEq/L (to one decimal place). Do not input
units on the Canvas answer, but they should be visible on your uploaded file.
Question 8
5 pts
189 mL of a 1.9 M solution of KBr is diluted to 668 mL. What is the new concentration of the solution (in M to 2 decimal
places)? Do not input units on the Canvas answer, but they should be visible on your uploaded file.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

