19.95 In the commercial preparation of aluminum, alu- minum oxide, Al,O3, is electrolyzed at 1000°C. (The mineral cryolite is added as a solvent.) Assume that the cathode reaction is Al+ + 3e¯→ Al How many coulombs of electricity are required to give 3.09 kg of aluminum?
19.95 In the commercial preparation of aluminum, alu- minum oxide, Al,O3, is electrolyzed at 1000°C. (The mineral cryolite is added as a solvent.) Assume that the cathode reaction is Al+ + 3e¯→ Al How many coulombs of electricity are required to give 3.09 kg of aluminum?
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter19: Electrochemistry
Section: Chapter Questions
Problem 19.95QP: In the commercial preparation of aluminum, aluminum oxide, Al2O3, is electrolyzed at 1000C. (The...
Related questions
Question
help
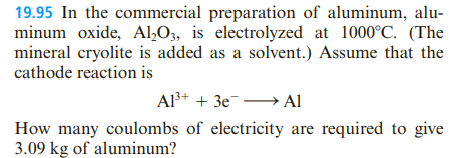
Transcribed Image Text:19.95 In the commercial preparation of aluminum, alu-
minum oxide, Al,O3, is electrolyzed at 1000°C. (The
mineral cryolite is added as a solvent.) Assume that the
cathode reaction is
Al+ + 3e¯→ Al
How many coulombs of electricity are required to give
3.09 kg of aluminum?
Expert Solution
Step 1
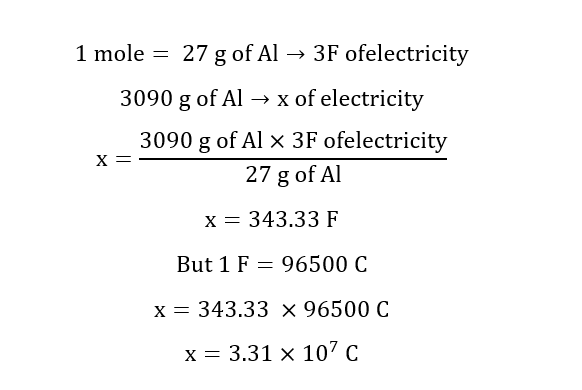
Given:
Mass of Al = 3.09 Kg = 3090 g
Reaction at cathode: Al+3 + 3e- → 3Al
Step 2
Calculation for amount of electricity required:
For 27 g of Aluminium 3F of electricity is required.
Step by step
Solved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning