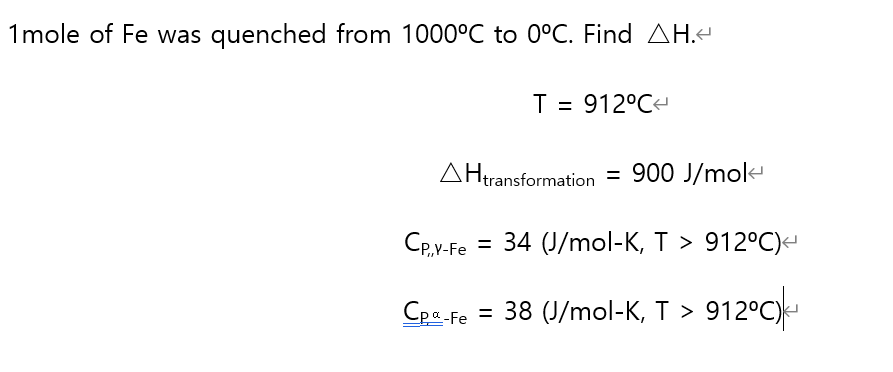
1mole of Fe was quenched from 1000°C to 0°C. Find AH.< T = 912°C< AHtransformation = 900 J/mol CP,Y-Fe = 34 (J/mol-K, T > 912°C)< Сpa-Fe = 38 (J/mol-K, T > 912°C)
Q: Outline reactions used in the preparation of the following compounds CeBr3 Pm2(SO4)3.8H2O…
A: The objective of this question is to show the synthesis involves the controlled transformation of…
Q: Draw all the possible monobromo - products for the following reaction? & G J Brz A 20
A: The reaction is an example of a free radical reaction. In the reaction, three steps are involved.…
Q: Which one of the H-C bonds indicated would have the lowest bond dissociation energy? OH-CH3 O…
A: The aim of this question is to explain bond dissoicton energy is the energy required to break a…
Q: (d) Predict the multiplicity observed for H1 in the ¹H NMR spectrum of 4ax and 4eq. Ignore couplings…
A: The objective of the question is to predict the multiplicity of H1 in the 1H NMR spectrum of 4ax and…
Q: a.Why can’t the elements of a compound be separated from one another by physical means? b.An…
A: The first part of the question is asking why the elements of a compound cannot be separated by…
Q: Look at the following orbital diagram. What principles/rules does it break? Explain. 1s 2s 2p
A: We have to find the principle broken in 1s22s22px2 configuration.
Q: The following activity data of component A have been obtained for liquid A-B solutions at 1500K. XA…
A: The aim of this question is to explain the observed activities with the calculated activities from…
Q: When the Hg2+ concentration is 6.95×10 M, the observed cell potential at 298K for an electrochemical…
A: Concentration of Hg2+ = 6.95 x 10-4 MEcell = 1.520 VE0Zn2+/Zn = −0.76 VE0Hg2+/Hg= 0.85 V
Q: Look at the following orbital diagram. What principles/rules does it break? Explain. 1s 2s 2p
A: We have to find the principle broken in 1s22s22px2 configuration.
Q: 7. Which of the following technologies are considered non-renewable energy sources? Choose ALL that…
A: The phenomenon of electrons occupying orbitals at the same energy sublevel singly before any orbital…
Q: Which of the following is true regarding the stereochemistry about the alkene bonds of structures A,…
A:
Q: 15. What products are formed from treatment of the enolate shown with the following substrates? ONa+…
A: Enolates are oxyallyl anions reagents that are used to make the substituted carbonyl compounds. The…
Q: preser Ca(NO3)2 is dissolved in water?
A: Option 3rd is the correct answer
Q: What is the correct lewis structure of 02 its paramagnetic property? considering (Has unpaired e-)
A: Lewis structure is a method of representing bonding pattern in a molecule.It tells us how different…
Q: (B) Relative intensity 100 50- 15 m/z 20 1. 40 55 60 71 80 86 (CH3)2CHCH₂CH₂NHCH₂CH₂CH(CH3)2 100…
A: The provided mass spectrum depicts the relative intensities of ion fragments at specific…
Q: electron-volt = (a) If an electron passes through an electrical potential difference of 1 V, it has…
A: 4.(a)According to the given data if the potential difference 1v then Energy is 1eVwavelength=0.225nm…
Q: 3. The following questions pertain to the two gas chromatograms of the same straight chain…
A: The objective of this question is to show the chromatogram A and broadening peaks indicate possible…
Q: Please follow the SCH4U rules for electron configurations and orbital diagrams
A: The objective of this question is to determine the number of bonds that Rhodium (Rh) can form…
Q: Ten grams of soil were displaced with 250 mL of 1 M ammonium acetate and made to a final volume of 1…
A: The aim of this question is to explain the estimated exchangeable cations in meq/100 g…
Q: Complete and balance the following redox reaction in basic solution MnO4 (aq) + C₂O4²-(aq) → MnO₂…
A: Oxidation is defined as an increase in the oxidation state of the element.It can also be defined as…
Q: Determine the time in seconds required to sputter 50 nm of Si using a 10 µA/cm2 beam of 45 keV ions…
A: The aim of this question is to explain the 50 nm Si sputtering using 10 µA/cm² 45 keV Ne, Kr and Xe…
Q: For the reaction H₂(e) + CO₂(e) = H₂O(g) + co(e) at 700 °C, K, -0.534. Calculate the number of moles…
A: Given:The reaction: Kc at 700 0C , Equilibrium constant KC = 0.534Initial moles of CO=0.170…
Q: The answer is D. Please explain. Thanks.
A: The objective of the question is to determine the signs of the change in Gibbs free energy (ΔG),…
Q: Consider a 25.00 mL solution that is 0.1000 M in V2+ titrated with a 0.1250 M standard Sn solution…
A: "Dear student, as an expert we are only allowed to answer one quesstion at a time and 3 sub-part at…
Q: Describe it in detail. 2. Describe "SP2 and SP3 bonding" and describe "crystal structure" according…
A: The objective of this question is to determine the variations in electronic configurations that lead…
Q: Gasoline samples containing pollutant S was analysed using GC-FID. The data of analysis was shown in…
A: Gasoline samples containing a pollutant S were analyzed. For injection 1:Peak area of internal…
Q: H₂O en Lotuinjs: COH НО НО
A: Mechanism represents the chemical reaction in which reactants change into desired products by using…
Q: If the rate of formation of ammonia is 0.345 M/s, what is the rate of disappearance of N₂? N₂(g) +…
A: Consider the given reaction is as follows;Given information:Rate of formation of ammonia = Rate of…
Q: The anode in the above cell can actually be considered a standard hydrogen electrode if…
A: The objective of this question is to show the cell configuration, the anode is a standard hydrogen…
Q: Band structure" of "Hatom chain" and "H mole chain" are drawn as "graphs" and "intermediate…
A: The aim of this question is to explain the band structure" of "Hatom chain" and "H mole chain" are…
Q: (A) 86α ULLE 58 72 60 Relative intensity 100- 15 m/z 20 40 80 100 114a Cattato co C3HCH₂N(CH3)…
A: Interpreting a mass spectrum involves identifying the main peaks and assigning them to specific…
Q: Relative intensity 100 50 15 mvz 20 & 41 40 57 60 68 81 80 85 (96) 100 OH H+ 194*. 120
A: The main aim of this question is to explain the mass spectrum peaks of given molecule. The mass…
Q: e Now consider the oxidation half-reaction. Enter the number of electrons needed to balance this…
A: The objective of the question is to determine the number of electrons needed to balance the…
Q: Fill in the missing information: symbol 2- Te atom or ion? check all that apply neutral atom neutral…
A: The objective of the question is to provide the missing information.
Q: 8. Show the products of the following enamine reactions. H&C CH₂ 1. 6 2 H₂C-N-CH₂ H₂C-N-CH, 2. HgO+…
A: This is an example of reaction of enamine with different electrophiles
Q: Fluorite (CaF2) has a face centered cubic structure, space group 225, with network parameter equal…
A: The objective of this question is to show the structure involves calcium ions at the 4a(0,0,0) site…
Q: Give the major product for the reaction below. A E F H HCI KMnO4, OH, heat H H₂O H₂ Pd D cold,…
A: Note: Since you have posted multiple questions with multiple sub-parts, we will provide the solution…
Q: 2) What is the relative error in delivering a volume of 18.28 mL from a class A 25 mL buret?…
A: The objective of the question is to determine the relative error of the given measurement.
Q: Br. Delta Draw all the possible monobromo - products for the following reaction? G Br₂
A: The given reaction is an example of a free radical reaction. In the presence of heat, halide…
Q: When the Pb²+ concentration is 5.35×104 M, the observed cell potential at 298K for an…
A: Given,
Q: Salt bridge TU A concentration cell similar to the one shown is composed of two Cr electrodes and…
A: The objective of this question is to calculate the cell potential for a concentration cell composed…
Q: 5) A certain aerobic organism is able to metabolize the following glycolipid: HO CH₂OH OH H OH A.…
A: The objective of this question is to show the concept of glycosidic bonds, hydrolysis, and the…
Q: Write the shorthand electron configuration for Rhodium
A: The shorthand electronic configuration of rhodium (Rh) is: [Kr]4d85s1
Q: how does the chemical structure look for propiophenone when it's melting point is 18.6 celsius?
A: The aim of this question is to explain the chemical structure look for propiophenone when it's…
Q: Table 1. Mass calculations for copper electrode. Mass of copper (initial) (g) Mass of copper (final)…
A: The objective of the first question is to calculate the partial pressure of the hydrogen gas that…
Q: Cu²+ A1³+ HS™ F CC14 Lewis Acid Lewis Base Can act as either a Le Acid or Lewis Base Neither a Lewis…
A: Given some cations and anions. Determine Lewis acid and base.
Q: Hydrofluoric acid and water react to form fluoride anion and hydronium cation, like this: HF(aq)…
A: The equilibrium constant (K) is a numerical value that expresses the ratio of the concentrations of…
Q: Choose a different family of natural products derived from the acetate pathway and propose how such…
A: The objective of this question is to show the different family of natural products derived from the…
Q: In a paragraph form, provide the experimental procedures with the laboratory equipments that needs…
A: The objective of this question is to explain the essential laboratory equipment are essential in…
Q: Which bond in the following molecule is the longest? H H-C- H 3 -CEC- 5 H -C-H H A) bond 5 B) bond 2…
A: We to find the longest bond in the given organic compound.
Step by step
Solved in 5 steps

- Help with the following question Ksp for ZnCO3 is 1.46 x 10 ^-10Hexanoic acid was added to an immiscible biphasic solvent sysem, water and CCl4 at 20.0OC and the equilibrium concentrations of hexanoic acid were determined to be 3.66 g/L in H2O and 67.0 g/L in CCl4. Caluclate the distrubution coeffiecent (D1) of hexanoic acid in CCl4 with respect to water.What is ΔHsys for a reaction at 51.4 °C with ΔSsurr = 749 J mol-1 K-1 ? Express your answer in kJ mol-1 to at least two significant figures.
- Aleks data for PbCO3 is 7.40 x 10^-14.What is ΔHsys for a reaction at 16.9 °C with ΔSsurr = -159 J mol-1 K-1 ? Express your answer in kJ mol-1 to at least two significant figures. (Please type answer no write by hend)Use the following equilibria2CH4(g) <----> C2H6(g) + H2(g) Kc1 = 9.5 × 10-13CH4(g) + H2O(g) CH3OH(g) + H2(g) Kc2 = 2.8 × 10-21to calculate the value of Kc' for the following reaction:2CH3OH(g) + H2(g) <---> C2H6(g) + 2H2O(g) Enter your response in scientific notation, e.g. enter 2E3 for 2000
- 9. Calculate the Go in kJ/mol. 3Fe2+ (s) + 2Cr(aq) ⟶2Cr3+(aq) + 3Fe (s); Eocell=0.30 V Group of answer choices -170 +170 +87 +58 -195 10. Calculate Ecell in volts. Zn(s)|Zn2+(2.50x10-4M)||Sn2+(1.50M)|Sn(s) Eocell=+0.624 V Group of answer choices 0.736 0.635 0.512 0.848 no correct answerKsp for Co(OH)2 is 5.92x10^-15Hexanoic acid was added to an immiscible biphasic solvent system, water and CCl4 at 20.0OC and the equilibrium concentrations of hexanoic acid were determined to be 3.66 g/L in H2O and 67.0 g/L in CCl4. Caluclate the distrubution coeffiecent (D2) of hexanoic acid in water with respect to CCl4.

