1o5 POH (c) What is the pH at the first equivalence point? (d) What volume of NaOH is needed to reach the second equivalence point and what species exist in solition at that pH? (e) What will the pH of the solution when 75 ml of NaOH has been added to the solution?
1o5 POH (c) What is the pH at the first equivalence point? (d) What volume of NaOH is needed to reach the second equivalence point and what species exist in solition at that pH? (e) What will the pH of the solution when 75 ml of NaOH has been added to the solution?
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter14: Equilibria In Acid-base Solutions
Section: Chapter Questions
Problem 74QAP: Fifty cm3 of 1.000 M nitrous acid is titrated with 0.850 M NaOH. What is the pH of the solution (a)...
Related questions
Question
100%
I know you guys can only answer one question at a time, could you answer c - e? I sent the set because the questions depend on each other. Thank you!
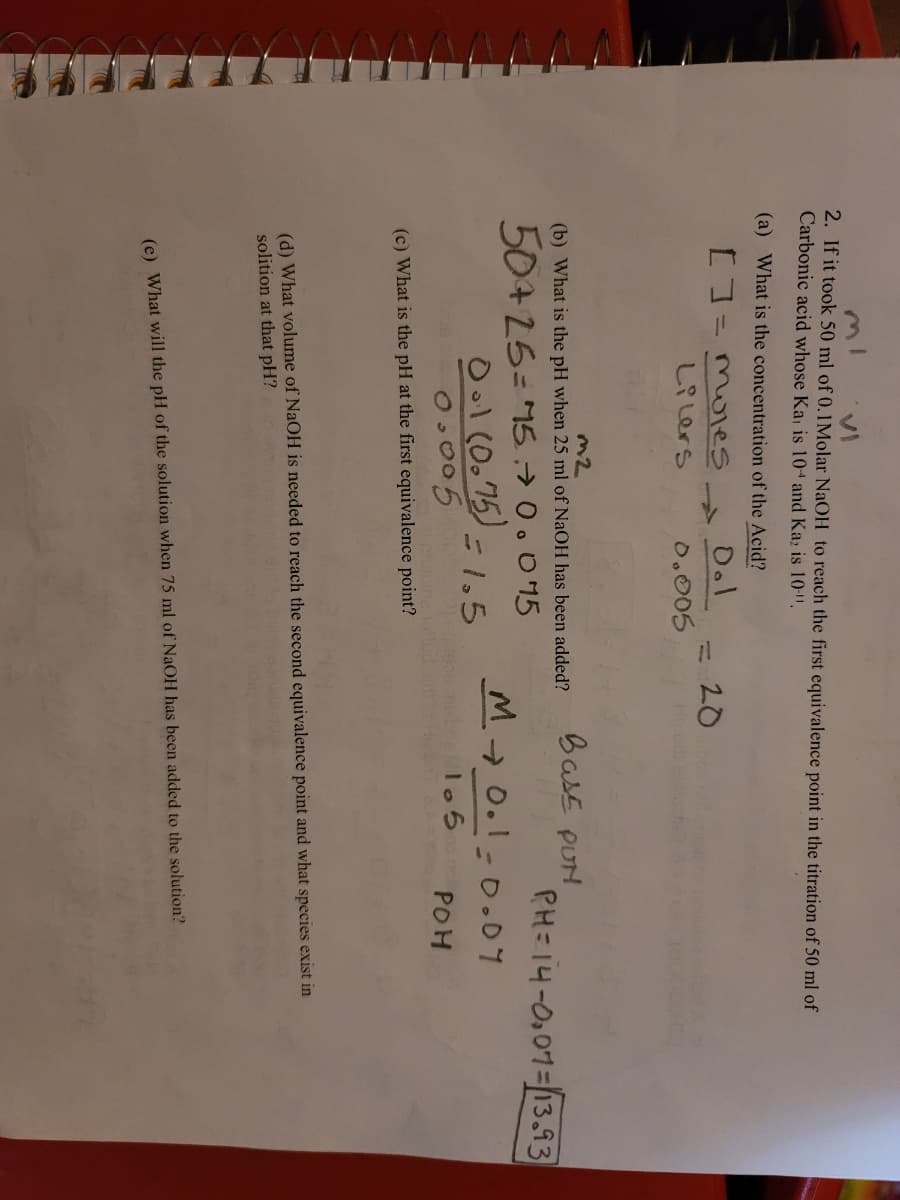
Transcribed Image Text:く
2. If it took 50 ml of 0.1Molar NaOH to reach the first equivalence point in the titration of 50 ml of
Carbonic acid whose Ka is 104 and Ka; is 10-".
(a) What is the concentration of the Acid?
D.l
20
しR Lers
0.005
Ho
BasE PUH
(b) What is the pH when 25 ml of NaOH has been added?
PH=14-0,01=13.93
50+25= 1570.095
Ool (0.75) =1,5
O.005
M 0.1-0.07
bcalo 5
POH
(c) What is the pH at the first equivalence point?
(d) What volume of NaOH is needed to reach the second equivalence point and what species exist in
solition at that pH?
(e) What will the pH of the solution when 75 ml of NaOH has been added to the solution?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 6 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning
