2 0/1 point Calculate AG, in kJ, for the dissolution equilibrium (equilibrium of a saturated solution) of CaF2 at 25°C. Enter your answer, rounded to the correct number of significant figures, into the box. Do not use scientific notation. If the answer is a decimal, include a O before the decimal point. If your answer is negative, include the negative sign with no space between it and the number. 491 For T=25.0°C, AG = KJ
2 0/1 point Calculate AG, in kJ, for the dissolution equilibrium (equilibrium of a saturated solution) of CaF2 at 25°C. Enter your answer, rounded to the correct number of significant figures, into the box. Do not use scientific notation. If the answer is a decimal, include a O before the decimal point. If your answer is negative, include the negative sign with no space between it and the number. 491 For T=25.0°C, AG = KJ
Chapter7: Statistical Data Treatment And Evaluation
Section: Chapter Questions
Problem 7.26QAP
Related questions
Question
Help answer.
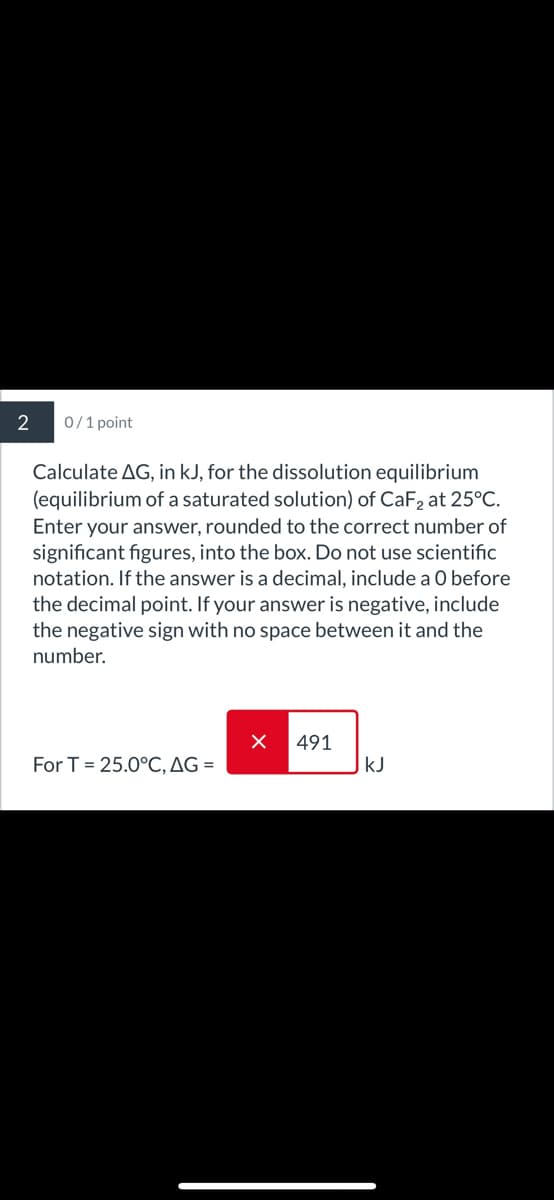
Transcribed Image Text:2
0/1 point
Calculate AG, in kJ, for the dissolution equilibrium
(equilibrium of a saturated solution) of CaF2 at 25°C.
Enter your answer, rounded to the correct number of
significant figures, into the box. Do not use scientific
notation. If the answer is a decimal, include a O before
the decimal point. If your answer is negative, include
the negative sign with no space between it and the
number.
491
For T=25.0°C, AG =
KJ
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

