2. A tank with a volume of 2.27 m³ is filled with an ideal gas at 20 °C to a pressure of 20,680 kPa. A. How many molecules of gas are in the tank? B. What would the pressure be if the tank and gas were left in the sun and warmed to 60 °C? C. Suppose the gas is transferred to a tank with a volume of 3.76 m³ and allowed to cool to 20 °C. What is the pressure of the gas now?
2. A tank with a volume of 2.27 m³ is filled with an ideal gas at 20 °C to a pressure of 20,680 kPa. A. How many molecules of gas are in the tank? B. What would the pressure be if the tank and gas were left in the sun and warmed to 60 °C? C. Suppose the gas is transferred to a tank with a volume of 3.76 m³ and allowed to cool to 20 °C. What is the pressure of the gas now?
College Physics
10th Edition
ISBN:9781285737027
Author:Raymond A. Serway, Chris Vuille
Publisher:Raymond A. Serway, Chris Vuille
Chapter10: Thermal Physics
Section: Chapter Questions
Problem 29P
Related questions
Question
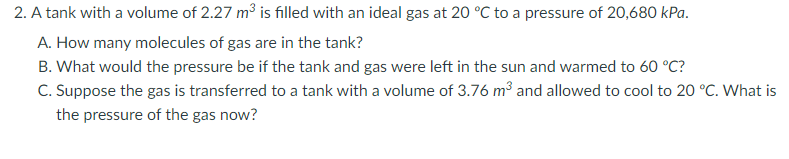
Transcribed Image Text:2. A tank with a volume of 2.27 m³ is filled with an ideal gas at 20 °C to a pressure of 20,680 kPa.
A. How many molecules of gas are in the tank?
B. What would the pressure be if the tank and gas were left in the sun and warmed to 60 °C?
C. Suppose the gas is transferred to a tank with a volume of 3.76 m³ and allowed to cool to 20 °C. What is
the pressure of the gas now?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

College Physics
Physics
ISBN:
9781285737027
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning


College Physics
Physics
ISBN:
9781285737027
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning
