2. Answer the following: A. Draw the molecular orbital diagram of the outermost shell of O2 B. Write the corresponding MO electron configuration C. Determine the bond order of O2
2. Answer the following: A. Draw the molecular orbital diagram of the outermost shell of O2 B. Write the corresponding MO electron configuration C. Determine the bond order of O2
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter10: Molecular Structure And Bonding Theories
Section: Chapter Questions
Problem 10.101QE: The molecular orbital diagram of NO shown in Figure 10.47 also applies to OF. Draw the complete...
Related questions
Question
answer #2
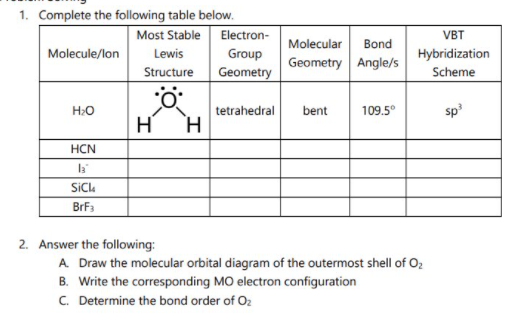
Transcribed Image Text:1. Complete the following table below.
Most Stable Electron-
Group
VBT
Molecular
Bond
Molecule/lon
Lewis
Hybridization
Geometry Angle/s
Structure
Geometry
Scheme
bent
109.5°
sp
H:O
tetrahedral
H
H.
HCN
SiCk
BrF3
2. Answer the following:
A. Draw the molecular orbital diagram of the outermost shell of Oz
B. Write the corresponding MO electron configuration
C. Determine the bond order of O2
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 2 images

Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
