2. As an example of microscopic thermodynamic analysis based on thermal-energy microstates, the figure to the right illustrates two systems (A and B), each containing two indistinguishable particles and possible energy levels from 1 to 8, with a particle residing in a given energy level having the same amount of energy as the level (e.g., a particle in energy-level 4 has 4 units of energy). The combined systems have a fixed total of 12 units of energy. Assuming that energy can be exchanged between the two systems, calculate the difference in entropy between condition #1 where the left-hand side has 8 units of energy and the right-hand side has 4 units of energy compared to condition #2 when the two sides reach their equilibrium condition. Note that the figure just shows one example of a configuration for condition #1. 8. 8 7 7 5 4 4 2 2 1 1
2. As an example of microscopic thermodynamic analysis based on thermal-energy microstates, the figure to the right illustrates two systems (A and B), each containing two indistinguishable particles and possible energy levels from 1 to 8, with a particle residing in a given energy level having the same amount of energy as the level (e.g., a particle in energy-level 4 has 4 units of energy). The combined systems have a fixed total of 12 units of energy. Assuming that energy can be exchanged between the two systems, calculate the difference in entropy between condition #1 where the left-hand side has 8 units of energy and the right-hand side has 4 units of energy compared to condition #2 when the two sides reach their equilibrium condition. Note that the figure just shows one example of a configuration for condition #1. 8. 8 7 7 5 4 4 2 2 1 1
Related questions
Question
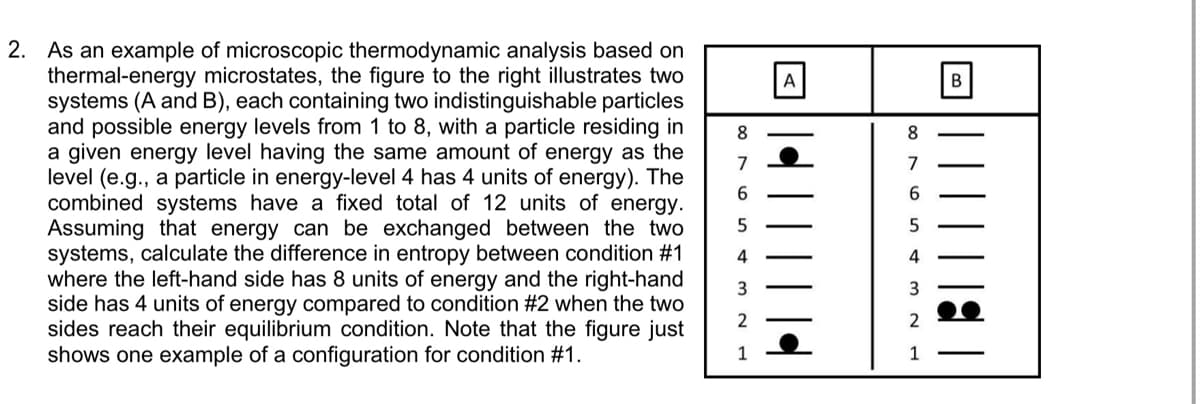
Transcribed Image Text:2. As an example of microscopic thermodynamic analysis based on
thermal-energy microstates, the figure to the right illustrates two
systems (A and B), each containing two indistinguishable particles
and possible energy levels from 1 to 8, with a particle residing in
a given energy level having the same amount of energy as the
level (e.g., a particle in energy-level 4 has 4 units of energy). The
combined systems have a fixed total of 12 units of energy.
Assuming that energy can be exchanged between the two
systems, calculate the difference in entropy between condition #1
where the left-hand side has 8 units of energy and the right-hand
side has 4 units of energy compared to condition #2 when the two
sides reach their equilibrium condition. Note that the figure just
shows one example of a configuration for condition #1.
8.
8
7
7
5
4
4
3
2
2
1
1
Expert Solution
Step 1
In both Conditions, 1st Calculate number of microstate of both system. Then put into entropy formula we get answer.
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images
