2. Calculate the normality and molarity of as solution containing 26.8 g of sodium carbonate, Na2CO3 per liter of solution 3. How many gram of concentrated sulphuric acid, H2SO4 are contained in 3.0 liters of o.800 N solution
2. Calculate the normality and molarity of as solution containing 26.8 g of sodium carbonate, Na2CO3 per liter of solution 3. How many gram of concentrated sulphuric acid, H2SO4 are contained in 3.0 liters of o.800 N solution
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter12: Solutions
Section: Chapter Questions
Problem 12.89QE
Related questions
Question
100%
please answer 2 and 3 with solution
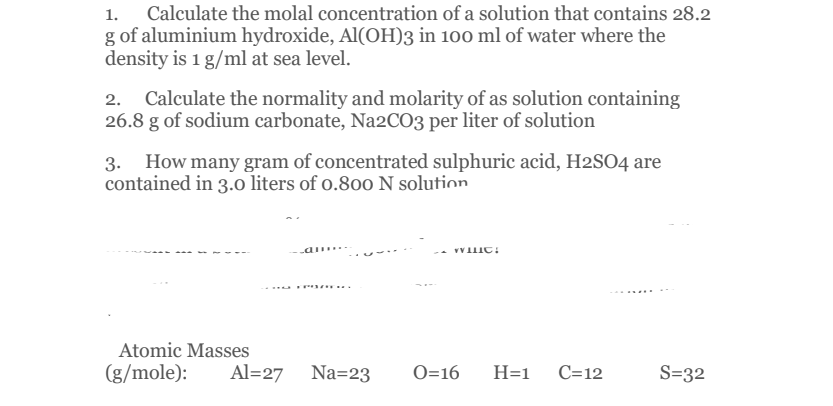
Transcribed Image Text:Calculate the molal concentration of a solution that contains 28.2
g of aluminium hydroxide, Al(OH)3 in 100 ml of water where the
density is 1 g/ml at sea level.
2. Calculate the normality and molarity of as solution containing
26.8 g of sodium carbonate, Na2CO3 per liter of solution
3. How many gram of concentrated sulphuric acid, H2SO4 are
contained in 3.0 liters of o.800 N solution
a .. -
T...
Atomic Masses
(g/mole):
Al=27 Na=23
O=16
H=1
C=12
S=32
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning