2. Let A = A(T,V) and G = G(T,p) be thermodynamic state functions with total differentials dA = -SdT – pdV and dG = -SdT+V dp, where p, V, T, and S are, respectively, pressure, volume, temperature and entropy. a) Use dA and suitable relationships between partial derivatives to show that Cv dT + ()av dS (2) T av av as ƏT where a 1 1 and Cy = T K = V ƏT V
2. Let A = A(T,V) and G = G(T,p) be thermodynamic state functions with total differentials dA = -SdT – pdV and dG = -SdT+V dp, where p, V, T, and S are, respectively, pressure, volume, temperature and entropy. a) Use dA and suitable relationships between partial derivatives to show that Cv dT + ()av dS (2) T av av as ƏT where a 1 1 and Cy = T K = V ƏT V
Physics for Scientists and Engineers
10th Edition
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Raymond A. Serway, John W. Jewett
Chapter20: The Kinetic Theory Of Gases
Section: Chapter Questions
Problem 45CP
Related questions
Question
I need help with my problem. I do not know how to approach it. Show me detailed steps.
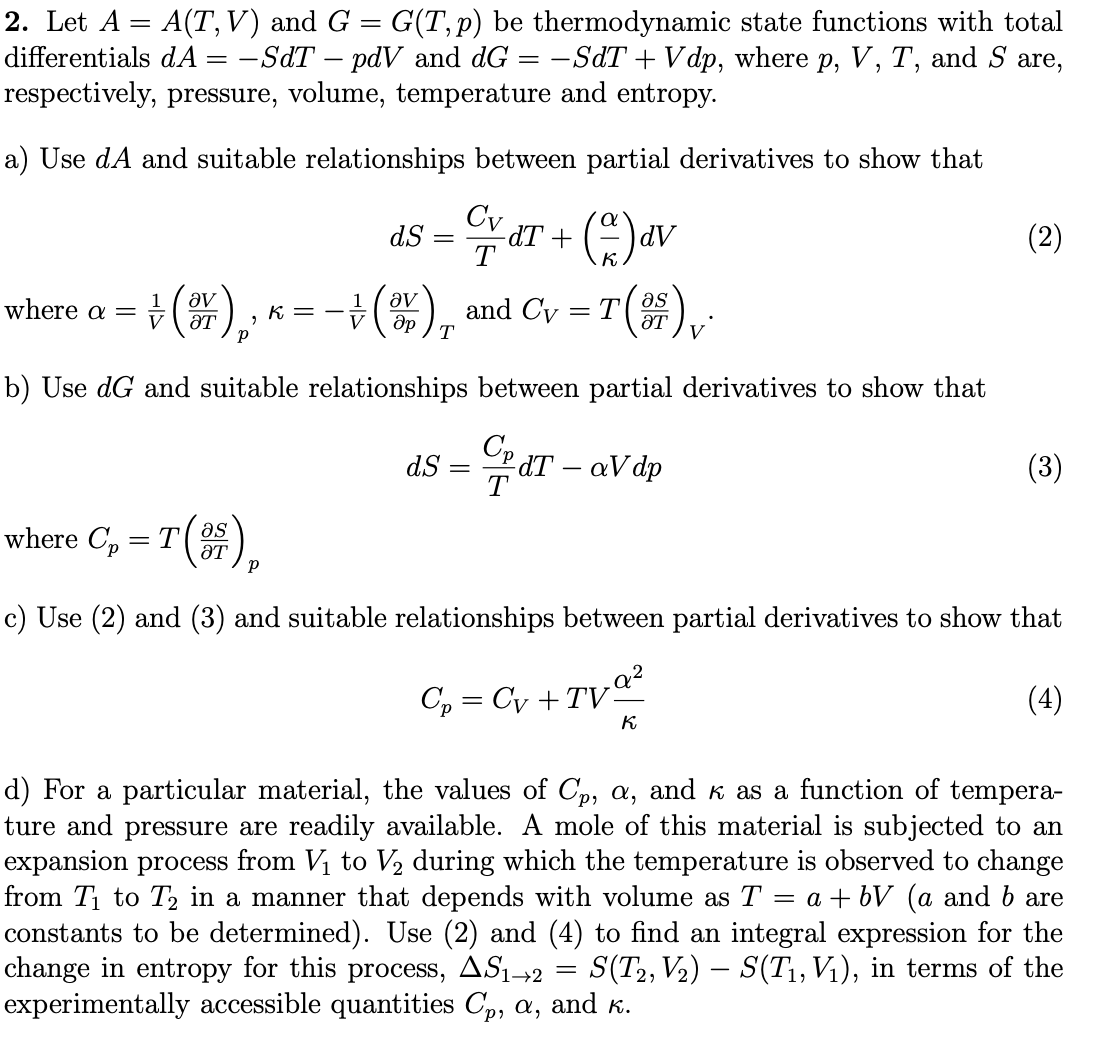
Transcribed Image Text:2. Let A = A(T,V) and G = G(T,p) be thermodynamic state functions with total
differentials dA = -SdT – pdV and dG = -SdT + V dp, where p, V, T, and S are,
respectively, pressure, volume, temperature and entropy.
a) Use dA and suitable relationships between partial derivatives to show that
Cv aT + ()av
dS
(2)
T
where a = +().,
÷(), and Cy = T()
1
1
K
ƏT
T
b) Use dG and suitable relationships between partial derivatives to show that
Cp
dT – aV dp
dS =
(3)
where C, = T().
as
ƏT
c) Use (2) and (3) and suitable relationships between partial derivatives to show that
Cp = Cy +TVª²
(4)
K
d) For a particular material, the values of Cp, a, and k as a function of tempera-
ture and pressure are readily available. A mole of this material is subjected to an
expansion process from V to V2 during which the temperature is observed to change
from T1 to T2 in a manner that depends with volume as T
constants to be determined). Use (2) and (4) to find an integral expression for the
change in entropy for this process, AS1→2 =
experimentally accessible quantities Cp, a, and K.
= a + bV (a and b are
S(T2, V2) – S(T1, Vị), in terms of the
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 8 steps with 8 images

Recommended textbooks for you

Physics for Scientists and Engineers
Physics
ISBN:
9781337553278
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers with Modern …
Physics
ISBN:
9781337553292
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781305952300
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning

Physics for Scientists and Engineers
Physics
ISBN:
9781337553278
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers with Modern …
Physics
ISBN:
9781337553292
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781305952300
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781285737027
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning

Physics for Scientists and Engineers, Technology …
Physics
ISBN:
9781305116399
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College