2. The Anemovax COVID-19 vaccine uses 0.950% (w/v) NaCl (normal saline, preservative- free) as its diluent. After removing from the freezer or refrigerator, the vaccine must be mixed with the diluent before administration. It is diluted to a total volume of 10.0 mL, and each vial contains eight doses. a. Calculate the mass (in grams) of NaCl that is needed in order to prepare a total of 10,000 doses of the vaccine. b. Express the concentration of the vaccine diluent in molarity. (MW = 58.44 g/mol)
2. The Anemovax COVID-19 vaccine uses 0.950% (w/v) NaCl (normal saline, preservative- free) as its diluent. After removing from the freezer or refrigerator, the vaccine must be mixed with the diluent before administration. It is diluted to a total volume of 10.0 mL, and each vial contains eight doses. a. Calculate the mass (in grams) of NaCl that is needed in order to prepare a total of 10,000 doses of the vaccine. b. Express the concentration of the vaccine diluent in molarity. (MW = 58.44 g/mol)
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter11: Intermolecular Forces And Liquids
Section11.6: Properties Of Liquids
Problem 2.2ACP
Related questions
Question
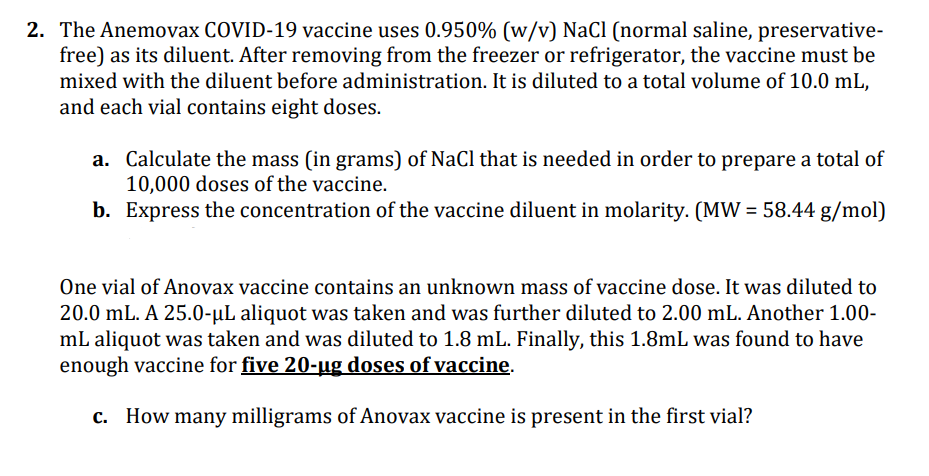
Transcribed Image Text:2. The Anemovax COVID-19 vaccine uses 0.950% (w/v) NaCl (normal saline, preservative-
free) as its diluent. After removing from the freezer or refrigerator, the vaccine must be
mixed with the diluent before administration. It is diluted to a total volume of 10.0 mL,
and each vial contains eight doses.
a.
Calculate the mass (in grams) of NaCl that is needed in order to prepare a total of
10,000 doses of the vaccine.
b. Express the concentration of the vaccine diluent in molarity. (MW = 58.44 g/mol)
One vial of Anovax vaccine contains an unknown mass of vaccine dose. It was diluted to
20.0 mL. A 25.0-µL aliquot was taken and was further diluted to 2.00 mL. Another 1.00-
mL aliquot was taken and was diluted to 1.8 mL. Finally, this 1.8mL was found to have
enough vaccine for five 20-ug doses of vaccine.
c. How many milligrams of Anovax vaccine is present in the first vial?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning