2. The five lowest energy levels of a certain atomic gas have the values E₁ = 0, E2 = 3.80 eV, E3= 4.30 eV, E4 = 7.2 eV, and E5 = 7.5 eV. (a) If the temperature is high enough that all levels are occupied and the gas is illuminated with light of wavelength 2400 nm, what transitions can occur? (b) Which of the transitions you found in part (a) will still occur if the temperature is so low that only the state E₁ is occupied? (c) What wavelength of the incident light would stimulate emission from the state Е₁?
2. The five lowest energy levels of a certain atomic gas have the values E₁ = 0, E2 = 3.80 eV, E3= 4.30 eV, E4 = 7.2 eV, and E5 = 7.5 eV. (a) If the temperature is high enough that all levels are occupied and the gas is illuminated with light of wavelength 2400 nm, what transitions can occur? (b) Which of the transitions you found in part (a) will still occur if the temperature is so low that only the state E₁ is occupied? (c) What wavelength of the incident light would stimulate emission from the state Е₁?
Related questions
Question
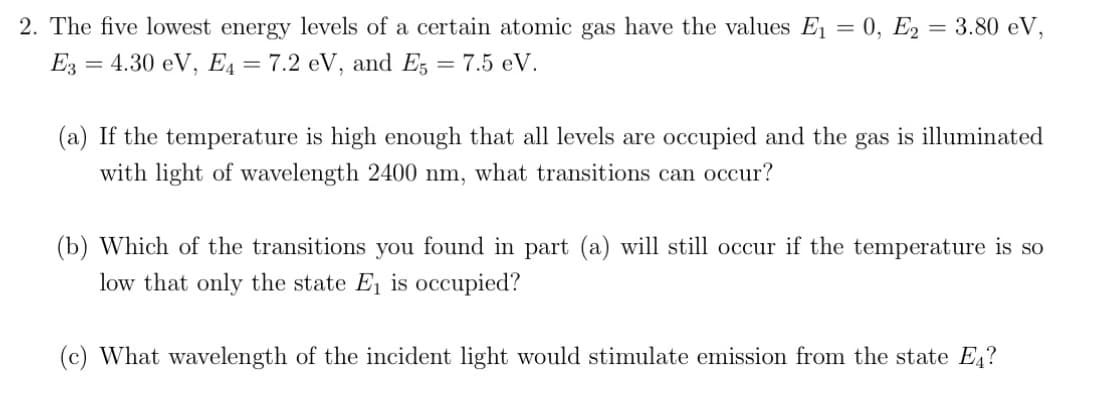
Transcribed Image Text:2. The five lowest energy levels of a certain atomic gas have the values E₁ = 0, E2= 3.80 eV,
E3 = 4.30 eV, E₁ = 7.2 eV, and E5 = 7.5 eV.
(a) If the temperature is high enough that all levels are occupied and the gas is illuminated
with light of wavelength 2400 nm, what transitions can occur?
(b) Which of the transitions you found in part (a) will still occur if the temperature is so
low that only the state E₁ is occupied?
(c) What wavelength of the incident light would stimulate emission from the state Е₁?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps
