2. The following reactions have been observed to be spontaneous: For each reaction, decide which OA is stronger and which RA is stronger. An example is shown. O ing idined alite 3* is geting RA: S'A + B+- A* + B Oxadized le a i OA: > > gtting C- 'C + A+ - C+ A OA: RA: > > B> ting onidied ahile Dt is redud. D+ +B D+ B+ geting. OA: 0+ RA: a) Construct a redox replacement series table for the four half-reactions represented above. Weakest OA Strongest RA Strongest OA Weakest RA b) Predict if the following would occur spontaneously in the direction written. 1. A + D+ D + A* (yes or no) 2. C + B C + B+ 3. D* + C D+ C
2. The following reactions have been observed to be spontaneous: For each reaction, decide which OA is stronger and which RA is stronger. An example is shown. O ing idined alite 3* is geting RA: S'A + B+- A* + B Oxadized le a i OA: > > gtting C- 'C + A+ - C+ A OA: RA: > > B> ting onidied ahile Dt is redud. D+ +B D+ B+ geting. OA: 0+ RA: a) Construct a redox replacement series table for the four half-reactions represented above. Weakest OA Strongest RA Strongest OA Weakest RA b) Predict if the following would occur spontaneously in the direction written. 1. A + D+ D + A* (yes or no) 2. C + B C + B+ 3. D* + C D+ C
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter18: Electrochemistry
Section: Chapter Questions
Problem 27Q: The free energy change for a reaction, G, is an extensive property. What is an extensive property?...
Related questions
Question
100%
Construct a redox replacement series table for the 4 half-reactions represented above. Predict if the following would occur in the direction written,
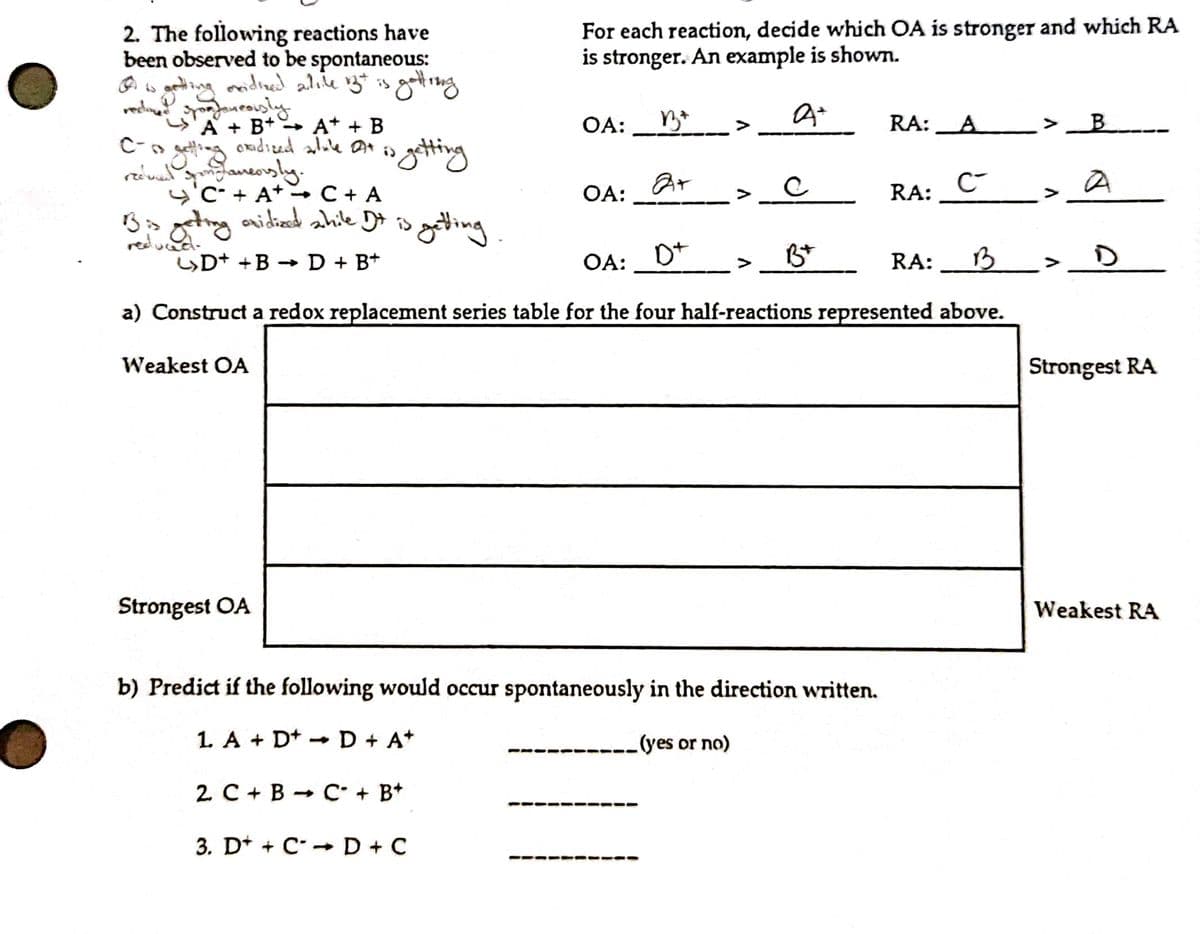
Transcribed Image Text:2. The following reactions have
been observed to be spontaneous:
For each reaction, decide which OA is stronger and which RA
is stronger. An example is shown.
s'A + B+→ A* + B
oxidized alale
RA: A > _B
.
OA:
getting
y'C + A* - C+ A
RA: _ C-
OA:
Bis ting anidized shile Dt is
redua-
D+ +B → D + B+
geting
OA: 0+
RA:
OA:
a) Construct a redox replacement series table for the four half-reactions represented above.
Weakest OA
Strongest RA
Strongest OA
Weakest RA
b) Predict if the following would occur spontaneously in the direction written.
1. A + D+ -D + A*
(yes or no)
2. C + B - C* + B+
3. D* + C -D +C
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning