2.26 Carbon dioxide (CO,) gas within a piston-cylinder assembly undergoes an expansion from a state where P 20 lbf/in., V 0.5 ft' to a state where p2-5 lbf/in., V 2.5 ft'. The relationship between pressure and volume during the process is p A + BV, where A and B are con- stants (a) For the CO, evaluate the work, in ft Ibf and Btu. (b) Evaluate A, in Ibf/in.", and B, in (Ibl/in."yft.
2.26 Carbon dioxide (CO,) gas within a piston-cylinder assembly undergoes an expansion from a state where P 20 lbf/in., V 0.5 ft' to a state where p2-5 lbf/in., V 2.5 ft'. The relationship between pressure and volume during the process is p A + BV, where A and B are con- stants (a) For the CO, evaluate the work, in ft Ibf and Btu. (b) Evaluate A, in Ibf/in.", and B, in (Ibl/in."yft.
Chapter3: The First Law Of Thermodynamics
Section: Chapter Questions
Problem 80AP: A car tile contains 0.0380 m3 of air at a pressure of 2.20105 Pa (about 32 psi). How much more...
Related questions
Question
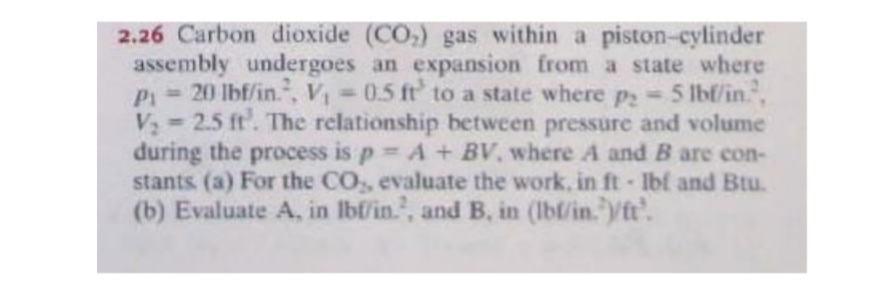
Transcribed Image Text:2.26 Carbon dioxide (CO,) gas within a piston-cylinder
assembly undergoes an expansion from a state where
P= 20 lbf/in., V 0.5 ft' to a state where p 5 1Ibf/in,
V 2.5 ft. The relationship between pressure and volume
during the process is p A + BV, where A and B are con-
stants (a) For the CO, evaluate the work, in ft Ibf and Btu.
(b) Evaluate A, in lbf/in., and B. in (lbl/in. ft'.
%3D
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you

