Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter5: Stoichiometry
Section: Chapter Questions
Problem 180IP: Tetrodotoxin is a toxic chemical found in fugu pufferfish, a popular but rare delicacy in Japan....
Related questions
Question
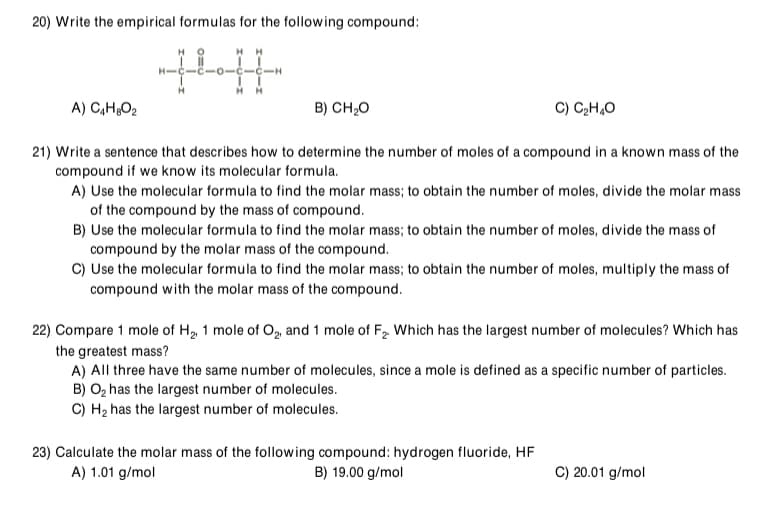
Transcribed Image Text:20) Write the empirical formulas for the following compound:
HH
-0-
A) C,H¿O2
B) CH,0
C) CH,O
21) Write a sentence that describes how to determine the number of moles of a compound in a known mass of the
compound if we know its molecular formula.
A) Use the molecular formula to find the molar mass; to obtain the number of moles, divide the molar mass
of the compound by the mass of compound.
B) Use the molecular formula to find the molar mass; to obtain the number of moles, divide the mass of
compound by the molar mass of the compound.
C) Use the molecular formula to find the molar mass; to obtain the number of moles, multiply the mass of
compound with the molar mass of the compound.
22) Compare 1 mole of H, 1 mole of O, and 1 mole of F2. Which has the largest number of molecules? Which has
the greatest mass?
A) All three have the same number of molecules, since a mole is defined as a specific number of particles.
B) O2 has the largest number of molecules.
C) H2 has the largest number of molecules.
23) Calculate the molar mass of the following compound: hydrogen fluoride, HF
A) 1.01 g/mol
B) 19.00 g/mol
C) 20.01 g/mol
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning