21.33 A certain hypothetical system is composed of 1 mole of identical, noninteracting, indistinguishable particles. Each particle has available to it only three energy levels, whose energies and degeneracies are e, =0, g,=1; ɛ,/k =100K , 82 = 3; e,/k= 300K, g,=5. (k is Boltzmann's constant.) (a) Calculate Z at 200 K. (b) Calculate the average number of molecules in each level at 200 K. (c) Calculate the average number of molecule in each level in the limit T ~ 00.
21.33 A certain hypothetical system is composed of 1 mole of identical, noninteracting, indistinguishable particles. Each particle has available to it only three energy levels, whose energies and degeneracies are e, =0, g,=1; ɛ,/k =100K , 82 = 3; e,/k= 300K, g,=5. (k is Boltzmann's constant.) (a) Calculate Z at 200 K. (b) Calculate the average number of molecules in each level at 200 K. (c) Calculate the average number of molecule in each level in the limit T ~ 00.
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter18: Nuclear Reactions
Section: Chapter Questions
Problem 65QAP
Related questions
Question
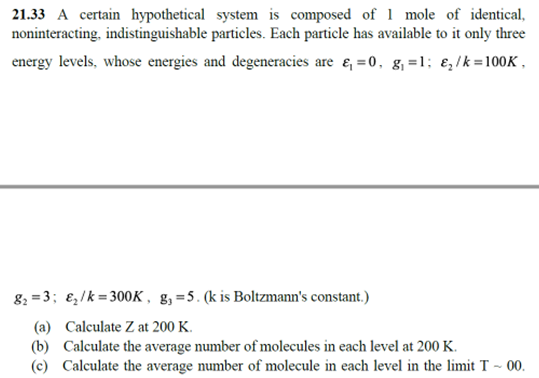
Transcribed Image Text:21.33 A certain hypothetical system is composed of 1 mole of identical,
noninteracting, indistinguishable particles. Each particle has available to it only three
energy levels, whose energies and degeneracies are e, =0, g,=1; ɛ,/k =100K ,
82 = 3; e,/k= 300K, g,=5. (k is Boltzmann's constant.)
(a) Calculate Z at 200 K.
(b) Calculate the average number of molecules in each level at 200 K.
(c) Calculate the average number of molecule in each level in the limit T ~ 00.
Expert Solution
Step by step
Solved in 10 steps with 10 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning