29.1 Quantization of Energy Planck's Contribution Energy is quantized in some systems, meaning that the system can have only certain energies and not a continuum of energies, unlike the classical case. This would be like having only certain speeds at which a car can travel because its kinetic energy can have only certain values. We also find that some forms of energy transfer take place with discrete lumps of energy. While most of us are familiar with the quantization of matter into lumps called atoms, molecules, and the like, we are less aware that energy. too, can be quantized. Some of the earliest clues about the necessity of quantum mechanics over classical physics came from the quantization of energy. This OpenStax book is available for free at http://cnx.org/contenticoll1406/1.9 Chapter 29 | Introduction to Quantum Physics 1145 14 V R L6000 K (white hot) 4000 K 3000 K (red hot) 1000 3000 A (nm) 2000 UV Visible IR range Figure 29.3 Graphs of blackbody radiation (from an ideal radiator) at three different radiator temperatures. The intensity or rate of radiation emission increases dramatically with temperature, and the peak of the spectrum shifts toward the visible and ultraviolet parts of the spectrum. The shape of the spectrum cannot be described with classical physics. EM radiation intensity
29.1 Quantization of Energy Planck's Contribution Energy is quantized in some systems, meaning that the system can have only certain energies and not a continuum of energies, unlike the classical case. This would be like having only certain speeds at which a car can travel because its kinetic energy can have only certain values. We also find that some forms of energy transfer take place with discrete lumps of energy. While most of us are familiar with the quantization of matter into lumps called atoms, molecules, and the like, we are less aware that energy. too, can be quantized. Some of the earliest clues about the necessity of quantum mechanics over classical physics came from the quantization of energy. This OpenStax book is available for free at http://cnx.org/contenticoll1406/1.9 Chapter 29 | Introduction to Quantum Physics 1145 14 V R L6000 K (white hot) 4000 K 3000 K (red hot) 1000 3000 A (nm) 2000 UV Visible IR range Figure 29.3 Graphs of blackbody radiation (from an ideal radiator) at three different radiator temperatures. The intensity or rate of radiation emission increases dramatically with temperature, and the peak of the spectrum shifts toward the visible and ultraviolet parts of the spectrum. The shape of the spectrum cannot be described with classical physics. EM radiation intensity
Related questions
Question
Quantization of Energy
• Explain Max Planck’s contribution to the development of
• Explain why atomic spectra indicate quantization.
Transcribed Image Text:29.1 Quantization of Energy
Planck's Contribution
Energy is quantized in some systems, meaning that the system can have only certain energies and not a continuum of energies,
unlike the classical case. This would be like having only certain speeds at which a car can travel because its kinetic energy can
have only certain values. We also find that some forms of energy transfer take place with discrete lumps of energy. While most of
us are familiar with the quantization of matter into lumps called atoms, molecules, and the like, we are less aware that energy.
too, can be quantized. Some of the earliest clues about the necessity of quantum mechanics over classical physics came from
the quantization of energy.
This OpenStax book is available for free at http://cnx.org/contenticoll1406/1.9
Chapter 29 | Introduction to Quantum Physics
1145
14 V R
L6000 K (white hot)
4000 K
3000 K (red hot)
1000
3000 A (nm)
2000
UV
Visible
IR
range
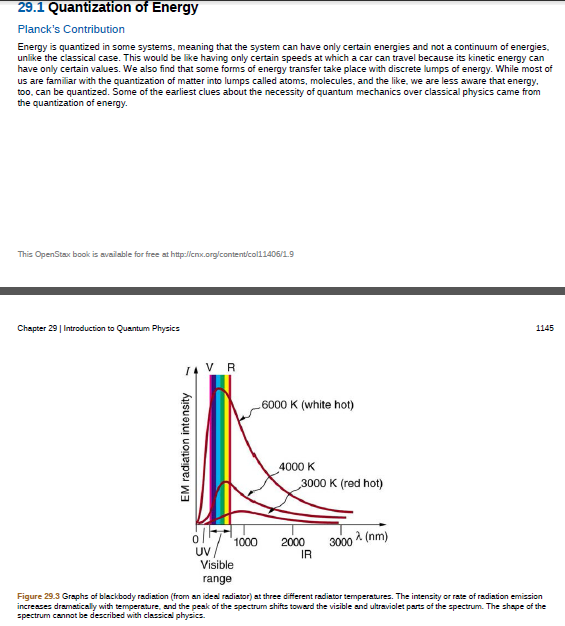
Figure 29.3 Graphs of blackbody radiation (from an ideal radiator) at three different radiator temperatures. The intensity or rate of radiation emission
increases dramatically with temperature, and the peak of the spectrum shifts toward the visible and ultraviolet parts of the spectrum. The shape of the
spectrum cannot be described with classical physics.
EM radiation intensity
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images
