29.8 The Particle-Wave Duality Reviewed Particle-wave duality-the fact that all particles have wave properties-is one of the cornerstones of quantum mechanics. We first came across it in the treatment of photons, those particles of EM radiation that exhibit both particle and wave properties, but not at the same time. Later it was noted that particles of matter have wave properties as well. The dual properties of particles and waves are found for all particles, whether massless like photons, or having a mass like electrons. (See Figure 29.28.) Figure 29.28 On a quantum-mechanical scale (i.e., very small), particles with and without mass have wave properties. For example, both electrons and photons have wavelengths but also behave as particles.
29.8 The Particle-Wave Duality Reviewed Particle-wave duality-the fact that all particles have wave properties-is one of the cornerstones of quantum mechanics. We first came across it in the treatment of photons, those particles of EM radiation that exhibit both particle and wave properties, but not at the same time. Later it was noted that particles of matter have wave properties as well. The dual properties of particles and waves are found for all particles, whether massless like photons, or having a mass like electrons. (See Figure 29.28.) Figure 29.28 On a quantum-mechanical scale (i.e., very small), particles with and without mass have wave properties. For example, both electrons and photons have wavelengths but also behave as particles.
Related questions
Question
The Particle-Wave Duality Reviewed
• Explain the concept of particle-wave duality, and its scope.
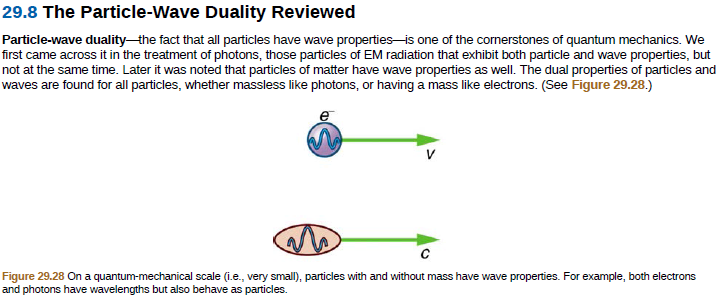
Transcribed Image Text:29.8 The Particle-Wave Duality Reviewed
Particle-wave duality-the fact that all particles have wave properties-is one of the cornerstones of quantum mechanics. We
first came across it in the treatment of photons, those particles of EM radiation that exhibit both particle and wave properties, but
not at the same time. Later it was noted that particles of matter have wave properties as well. The dual properties of particles and
waves are found for all particles, whether massless like photons, or having a mass like electrons. (See Figure 29.28.)
Figure 29.28 On a quantum-mechanical scale (i.e., very small), particles with and without mass have wave properties. For example, both electrons
and photons have wavelengths but also behave as particles.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images
