2A gas that has a volume of 98 liters, a temperature of 45 °C and an unknown pressure has its volume decreased to 34 liters and its temperature decreased to 35 °C. If the pressure after the change is 2.0 atm, what was the original pressure of the gas?
2A gas that has a volume of 98 liters, a temperature of 45 °C and an unknown pressure has its volume decreased to 34 liters and its temperature decreased to 35 °C. If the pressure after the change is 2.0 atm, what was the original pressure of the gas?
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter5: Gases
Section: Chapter Questions
Problem 5.44PAE
Related questions
Question
Number 12 plz
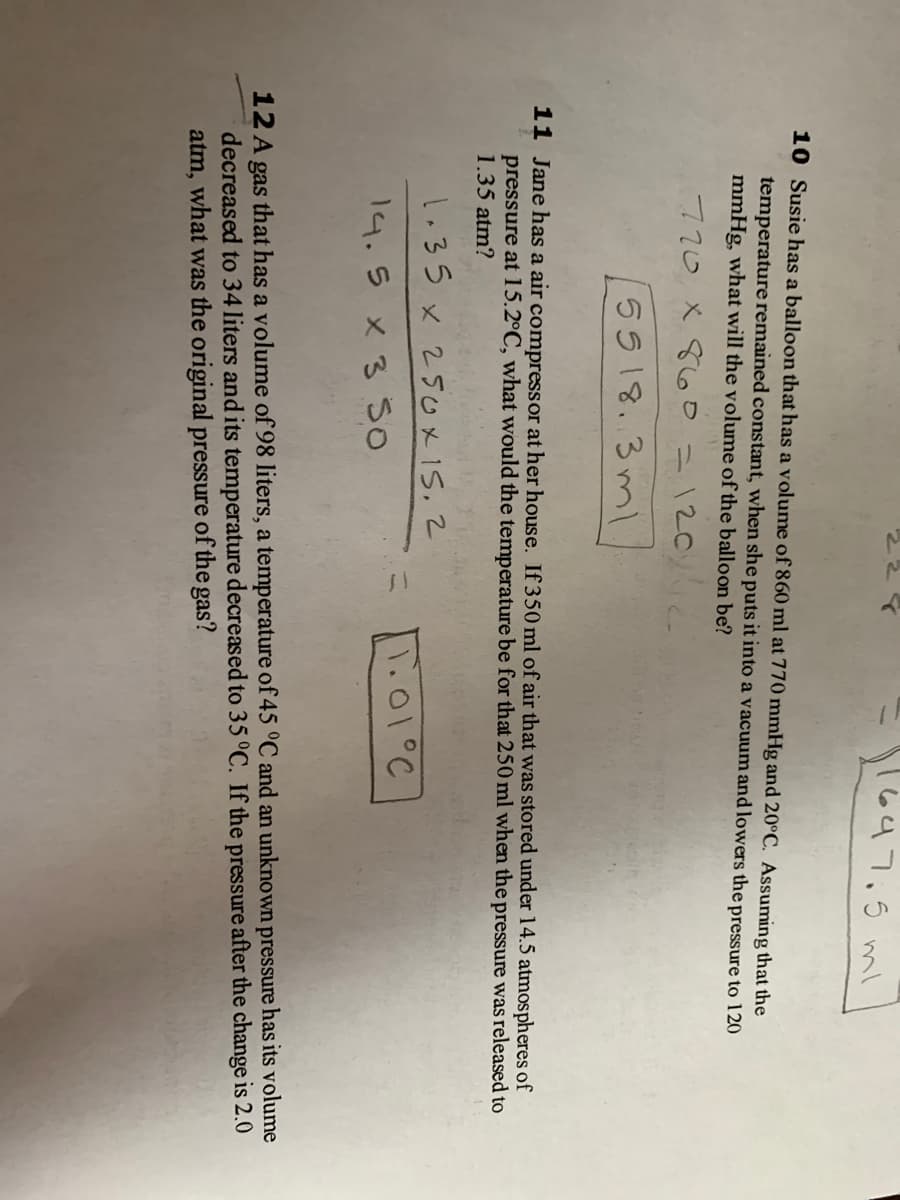
Transcribed Image Text:1647.5 ml
10 Susie has a balloon that has a volume of 860 ml at 770 mmHg and 20°C. Assuming that the
temperature remained constant, when she puts it into a vacuum and lowers the pressure to 120
mmHg, what will the volume of the balloon be?
770 x 8oo=120
5518. 3 ml
11 Jane has a air compressor at her house. If 350 ml of air that was stored under 14.5 atmospheres of
pressure at l15.2°C, what would the temperature be for that 250 ml when the pressure was released to
1.35 atm?
1.35 x 25OXIS. 2
T.01°C
14.5 x 3 so
12 A gas that has a volume of 98 liters, a temperature of45 °C and an unknown pressure has its volume
decreased to 34 liters and its temperature decreased to 35 °C. If the pressure after the change is 2.0
atm, what was the original pressure of the gas?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning