Introduction to General, Organic and Biochemistry
11th Edition
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Chapter9: Nuclear Chemistry
Section: Chapter Questions
Problem 9.10P: 9-10 Microwaves are a form of electromagnetic radiation that is used for the rapid heating of foods....
Related questions
Question
100%
Calculate the standard reaction free energy delta G for the following redox reaction . Round your answer to three sig figs. 
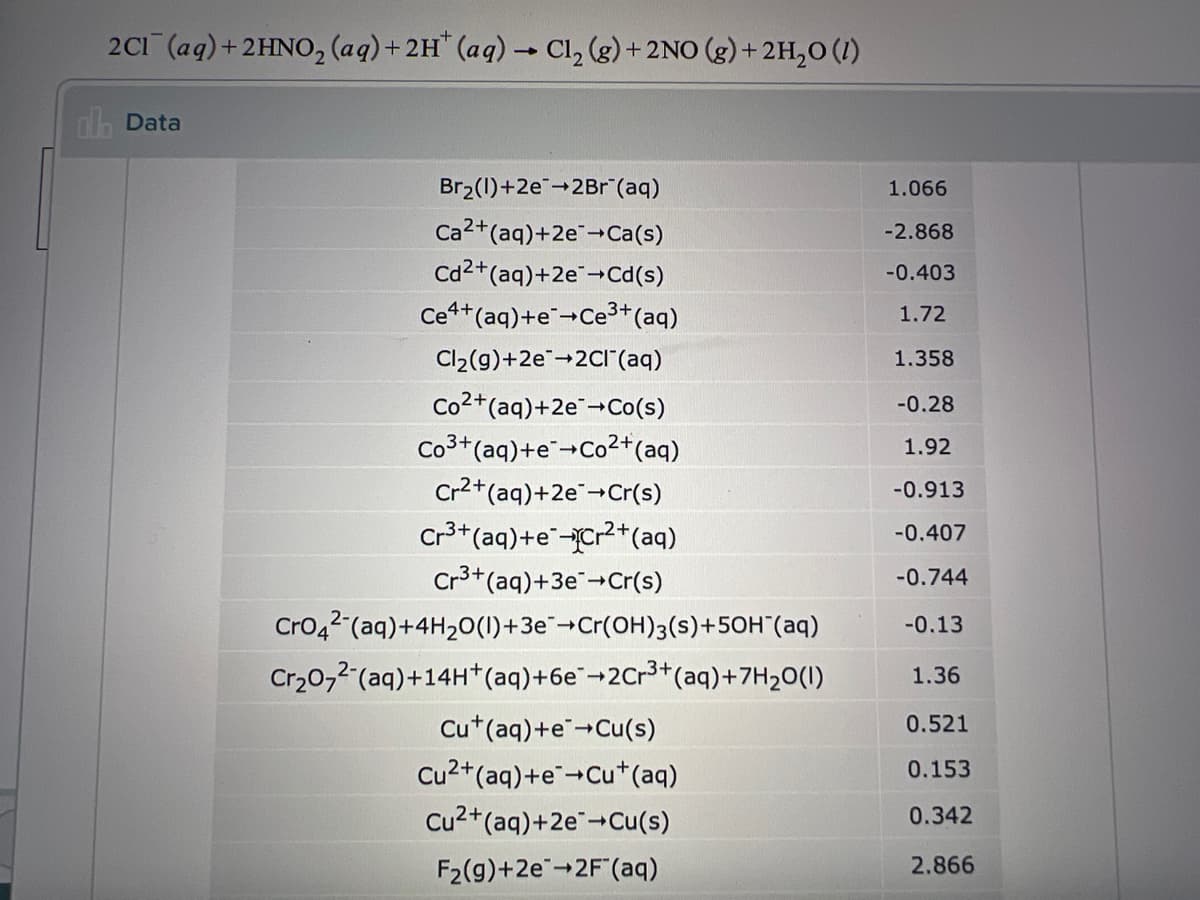
Transcribed Image Text:2C1 (aq)+2HNO, (aq)+ 2H" (aq) Cl, (g) + 2NO (g) +2H,0 (1)
ola Data
Br2(1)+2e-2Br"(aq)
1.066
Ca2+(aq)+2e-Ca(s)
Cd2+(aq)+2e-Cd(s)
Ce4+(aq)+e+Ce3+*(aq)
-2.868
-0.403
1.72
Cl2(g)+2e-2CI (aq)
1.358
Co2+(aq)+2e+Co(s)
-0.28
Co3+(aq)+e-Co2+(aq)
Cr2+(aq)+2e+Cr(s)
1.92
-0.913
Cr3+(aq)+e-Cr2+(aq)
Cr3+(aq)+3e+Cr(s)
-0.407
-0.744
Cro42 (aq)+4H20(1)+3e¯¬Cr(OH)3(s)+50H"(aq)
Cr20,2 (aq)+14H*(aq)+6e¯¬2Cr3+(aq)+7H20(1)
-0.13
1.36
Cu*(aq)+e"-Cu(s)
0.521
Cu2+(aq)+e+Cu*(aq)
0.153
Cu2+(aq)+2e+Cu(s)
0.342
F2(g)+2e-2F"(aq)
2.866
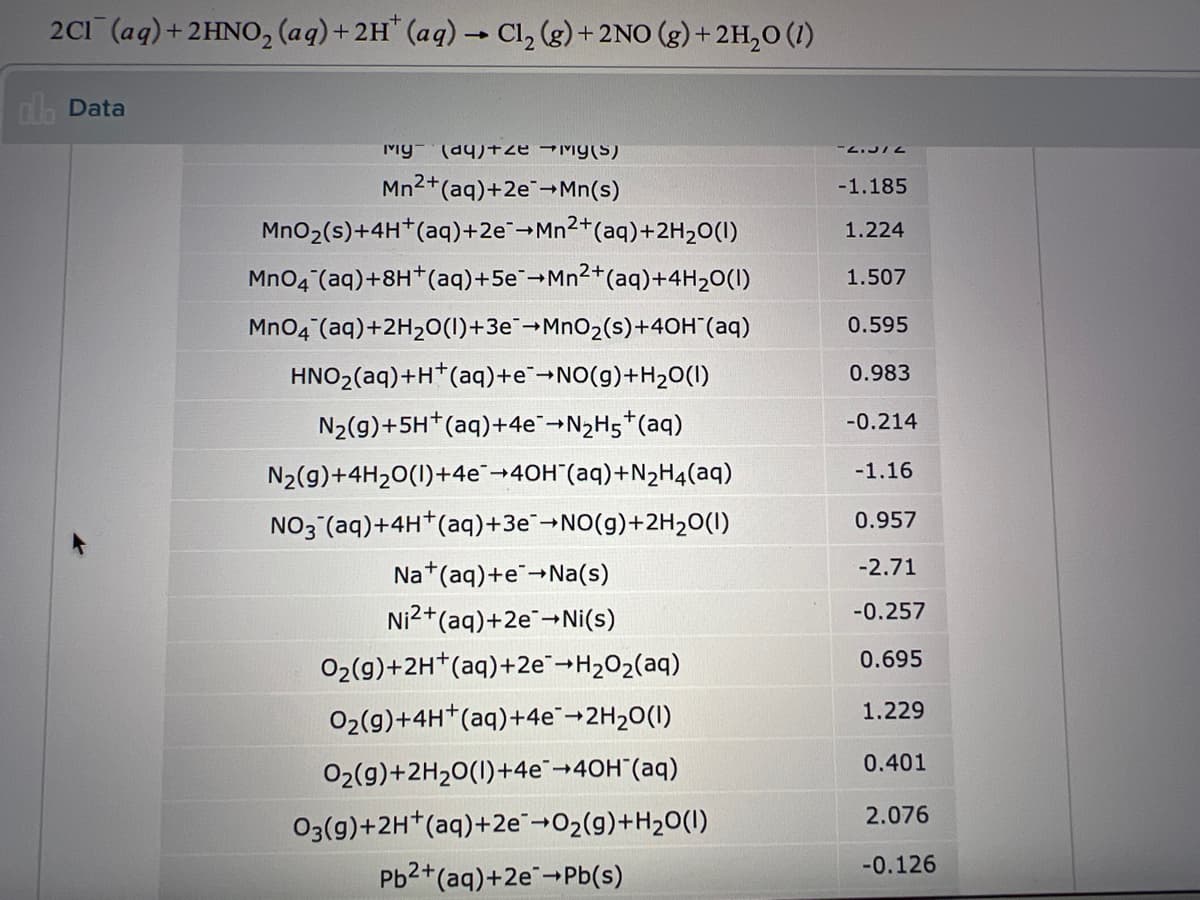
Transcribed Image Text:2C1 (aq)+2HNO, (aq)+2H" (aq) - Cl, (g) + 2NO (g) + 2H,0 (1)
dh Data
My- (a9)+ ze →My(S)
Mn2+(aq)+2e-Mn(s)
-1.185
MnO2(s)+4H*(aq)+2e¯¬Mn2+(aq)+2H2O(I)
1.224
Mno4 (aq)+8H*(aq)+5e-Mn2+(aq)+4H20(1)
1.507
MnO4 (aq)+2H20(1)+3e¯¬MnO2(s)+40H (aq)
0.595
HNO2(aq)+H*(aq)+E¯¬NO(g)+H20(1)
0.983
N2(g)+5H*(aq)+4e-N2H5*(aq)
-0.214
N2(g)+4H20(1)+4e+40H"(aq)+N2H4(aq)
-1.16
NO3 (aq)+4H*(aq)+3€¯¬NO(g)+2H20(1)
0.957
Na*(aq)+e→Na(s)
-2.71
Ni2+(aq)+2e-Ni(s)
-0.257
02(9)+2H*(aq)+2e-H2O2(aq)
0.695
1.229
02(9)+4H*(aq)+4e"-2H20(1)
0.401
02(9)+2H20(1)+4€→40H"(aq)
2.076
O3(g)+2H*(aq)+2e"-02(g)+H20(I)
-0.126
Pb2+(aq)+2e-Pb(s)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning