Chapter4: Least-squares And Calibration Methods
Section: Chapter Questions
Problem 1P
Related questions
Question
I need help finding the net ionic equation based off of the molecular equation in the previous question.
Help to solve Part B.
Transcribed Image Text:Muhammad
plution Reactions
1 of 16
<>
Review | Constants | Periodic Table
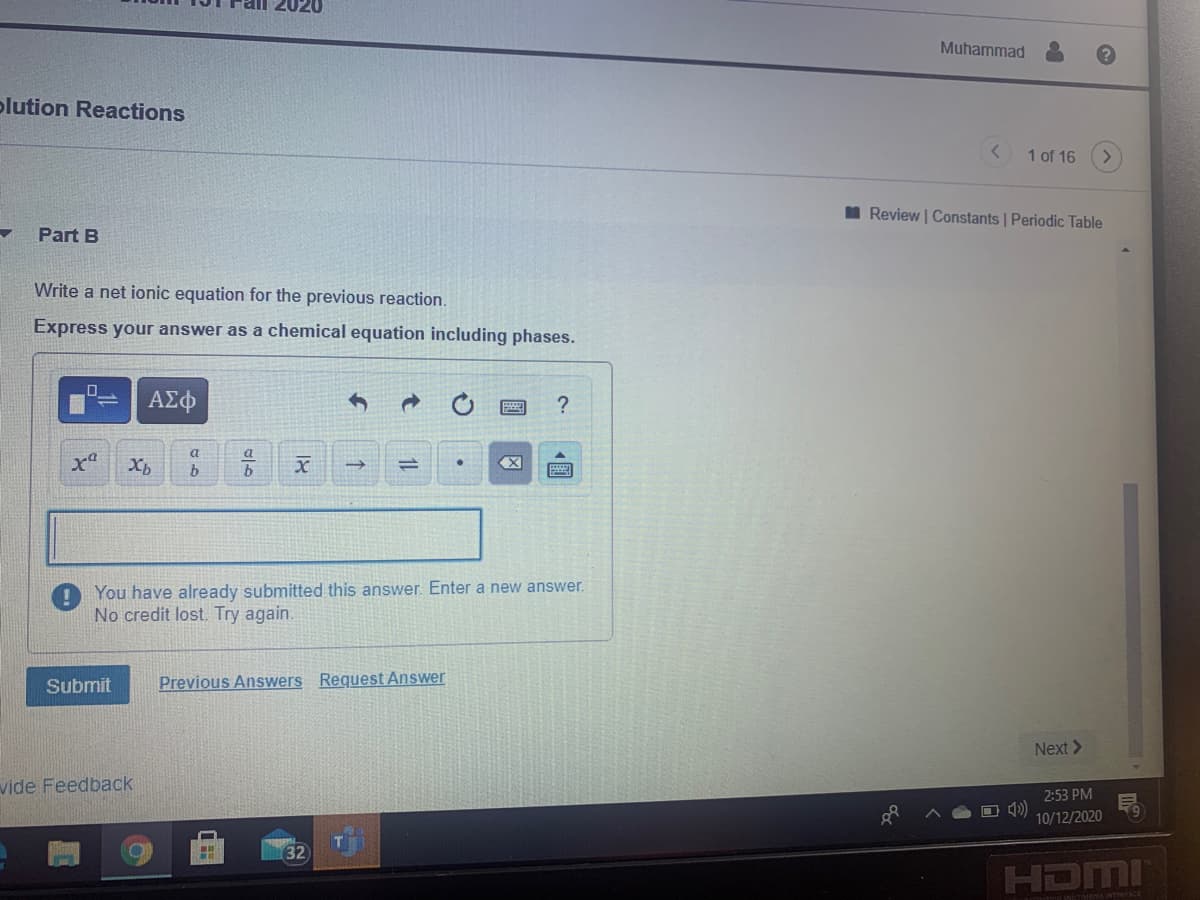
Part B
Write a net ionic equation for the previous reaction.
Express your answer as a chemical equation including phases.
ΑΣφ
a
You have already submitted this answer. Enter a new answer.
No credit lost. Try again.
Submit
Previous Answers Request Answer
Next >
vide Feedback
2:53 PM
O 4)
10/12/2020
32
HDMI
Transcribed Image Text:District
Destiny
A Rancho Pico
Freedom Speech -..
Chem 151 Fall 2020
Muhammad
Evolution Reactions
1 of 16
M Review | Constants | Periodic Table
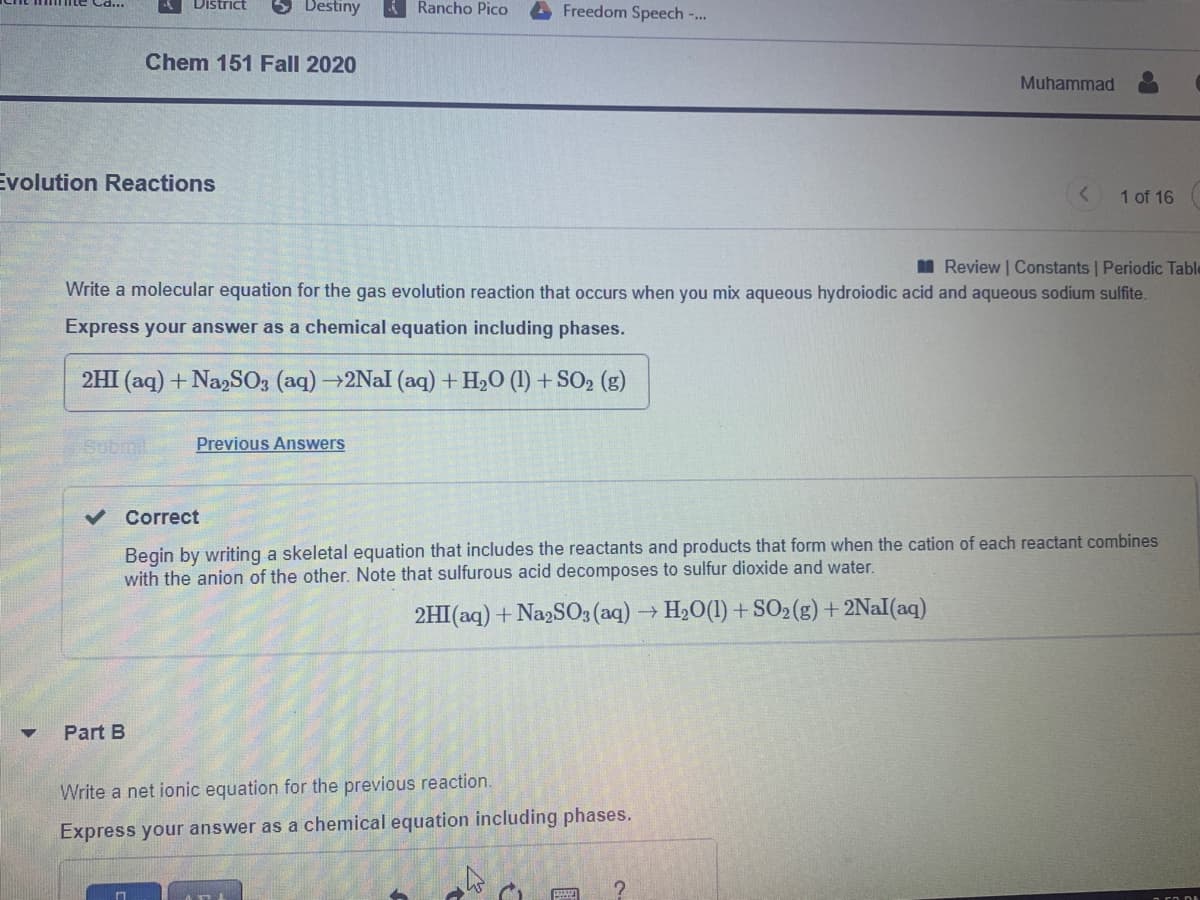
Write a molecular equation for the gas evolution reaction that occurs when you mix aqueous hydroiodic acid and aqueous sodium sulfite.
Express your answer as a chemical equation including phases.
2HI (aq) + NazSO3 (aq) →2Nal (aq) + H,O (1) + SO2 (g)
Previous Answers
Correct
Begin by writing a skeletal equation that includes the reactants and products that form when the cation of each reactant combines
with the anion of the other. Note that sulfurous acid decomposes to sulfur dioxide and water.
2HI(aq) + Na2SO3 (aq) → H20(1) + SO2(g) + 2NAI(aq)
Part B
Write a net ionic equation for the previous reaction.
Express your answer as a chemical equation including phases.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



