3 Charge Deflector E beam L An experiment to look for a charge imbalance between the proton and electron is constructed as shown. A beam of particles moving at speed vo = 1.0 x 106 m/s is prepared in an accelerator. Before striking the detector, the beam passes through a region of length L = 10m that contains a uniform electric field of strength & = 100 N/C perpendicular to the beam. (a) Where would a proton from the beam be expected to strike the detector? (L.e., how many meters above or below the beam line would it strike?) (b) Where would an electron from the beam be expected to strike the detector? (L.e., how many meters above or below the beam line would it strike?) (c) We do not believe a hydrogen atom has any charge, but an experiment can only put an upper limit on the net charge-not prove that it is zero. Suppose the detector in this experiment is unable to detect a deflection less than 1 nm. Le., if the deflection is 1 nm or less, the detector will register "0" deflection. If no deflection of hydrogen atoms is detected, what is the upper limit on the net charge of a hydrogen atom? In other words, what is the largest possible charge a hydrogen atom could have, if it is deflected by less than 1 nm? Express your result in coulombs (C) and as a fraction of the proton charge (e). This is the maximum difference between the electron and proton charge that is consistent with the experimental measurement. detector
3 Charge Deflector E beam L An experiment to look for a charge imbalance between the proton and electron is constructed as shown. A beam of particles moving at speed vo = 1.0 x 106 m/s is prepared in an accelerator. Before striking the detector, the beam passes through a region of length L = 10m that contains a uniform electric field of strength & = 100 N/C perpendicular to the beam. (a) Where would a proton from the beam be expected to strike the detector? (L.e., how many meters above or below the beam line would it strike?) (b) Where would an electron from the beam be expected to strike the detector? (L.e., how many meters above or below the beam line would it strike?) (c) We do not believe a hydrogen atom has any charge, but an experiment can only put an upper limit on the net charge-not prove that it is zero. Suppose the detector in this experiment is unable to detect a deflection less than 1 nm. Le., if the deflection is 1 nm or less, the detector will register "0" deflection. If no deflection of hydrogen atoms is detected, what is the upper limit on the net charge of a hydrogen atom? In other words, what is the largest possible charge a hydrogen atom could have, if it is deflected by less than 1 nm? Express your result in coulombs (C) and as a fraction of the proton charge (e). This is the maximum difference between the electron and proton charge that is consistent with the experimental measurement. detector
Glencoe Physics: Principles and Problems, Student Edition
1st Edition
ISBN:9780078807213
Author:Paul W. Zitzewitz
Publisher:Paul W. Zitzewitz
Chapter21: Electric Fields
Section: Chapter Questions
Problem 103A
Related questions
Question
100%
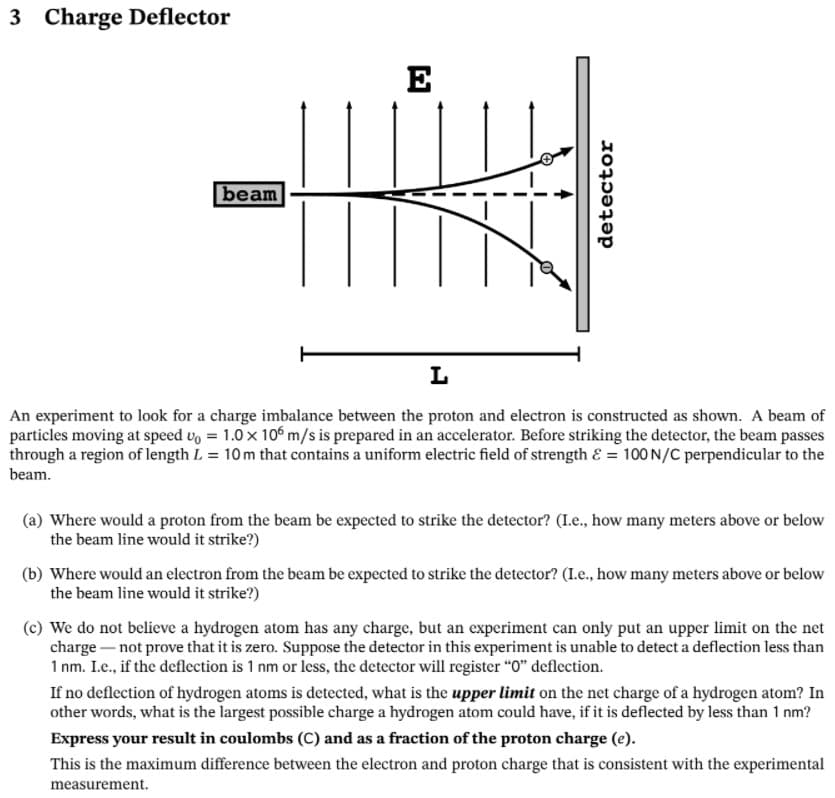
Transcribed Image Text:3 Charge Deflector
E
beam
An experiment to look for a charge imbalance between the proton and electron is constructed as shown. A beam of
particles moving at speed vo = 1.0 x 106 m/s is prepared in an accelerator. Before striking the detector, the beam passes
through a region of length L = 10 m that contains a uniform electric field of strength & = 100 N/C perpendicular to the
beam.
(a) Where would a proton from the beam be expected to strike the detector? (L.e., how many meters above or below
the beam line would it strike?)
(b) Where would an electron from the beam be expected to strike the detector? (L.e., how many meters above or below
the beam line would it strike?)
(c) We do not believe a hydrogen atom has any charge, but an experiment can only put an upper limit on the net
charge - not prove that it is zero. Suppose the detector in this experiment is unable to detect a deflection less than
1 nm. I.c., if the deflection is 1 nm or less, the detector will register "0" deflection.
If no deflection of hydrogen atoms is detected, what is the upper limit on the net charge of a hydrogen atom? In
other words, what is the largest possible charge a hydrogen atom could have, if it is deflected by less than 1 nm?
Express your result in coulombs (C) and as a fraction of the proton charge (e).
This is the maximum difference between the electron and proton charge that is consistent with the experimental
measurement.
detector
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps with 5 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you

Glencoe Physics: Principles and Problems, Student…
Physics
ISBN:
9780078807213
Author:
Paul W. Zitzewitz
Publisher:
Glencoe/McGraw-Hill

College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College

Physics for Scientists and Engineers, Technology …
Physics
ISBN:
9781305116399
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Glencoe Physics: Principles and Problems, Student…
Physics
ISBN:
9780078807213
Author:
Paul W. Zitzewitz
Publisher:
Glencoe/McGraw-Hill

College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College

Physics for Scientists and Engineers, Technology …
Physics
ISBN:
9781305116399
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781285737027
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning


College Physics
Physics
ISBN:
9781305952300
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning