Q: Consider the balanced chemical reaction below. How many grams of CaSO, can be produced from 1.00 kg…
A:
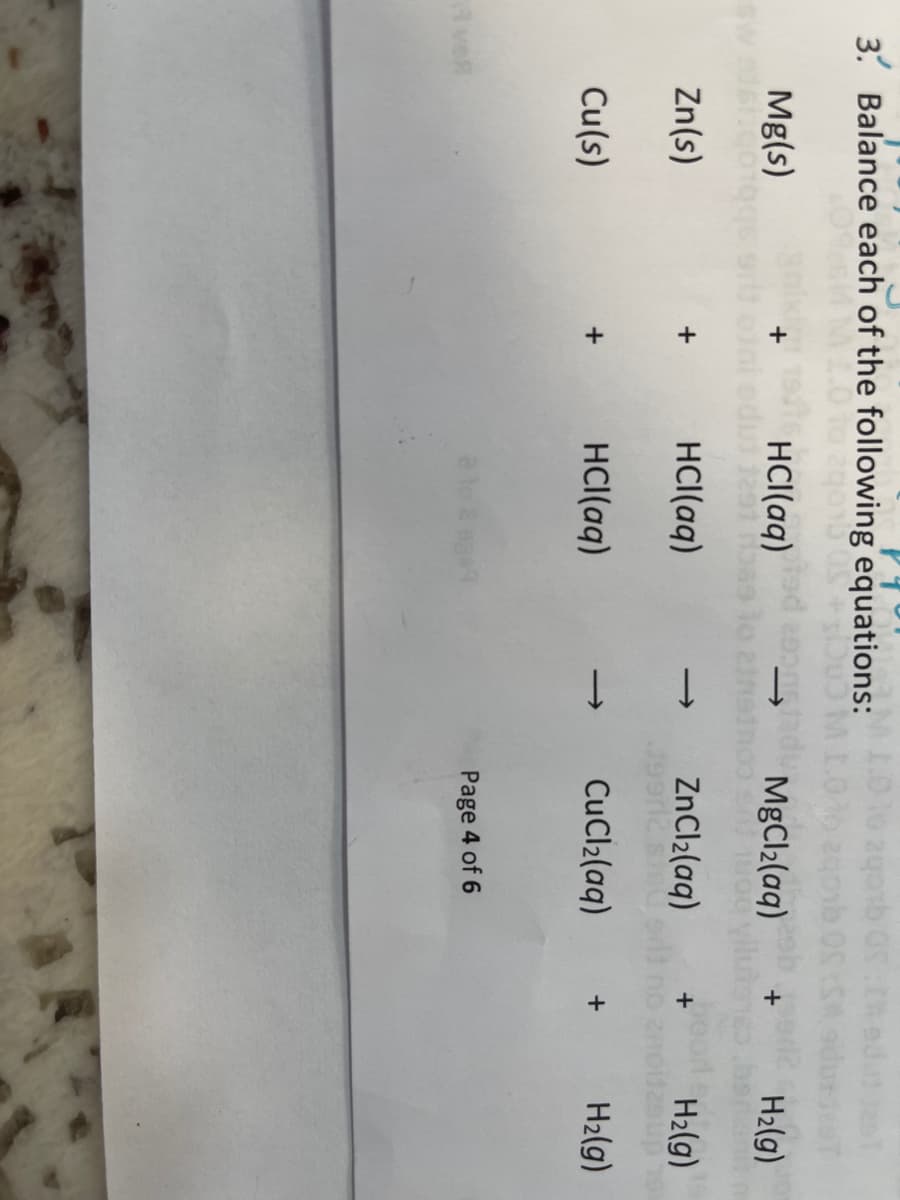
Q: Chemistry Balance the following equations: a) NH3 (aq) + O2 (aq) NO (g) + H2O (l) (2) b) Fe2O3 (s)…
A:
Q: 18.1 g of ammonia gas reacts with 149 g copper (II) oxide to form 4.80 g nitrogen gas, elemental…
A: Given : Mass of ammonia i.e NH3 taken = 18.1 g Mass of copper (II) oxide i.e CuO taken = 149 g And…
Q: Question 11
A: Total number of shared electrons in acetic acid has to be calculated.
Q: When silver nitrate reacts with copper(II) chloride, silver chloride and copper(II) nitrate are…
A: Given balanced equation, 2AgNO3(aq) + CuCl2(s) → 2AgCl(s) + Cu(NO3)2(aq)
Q: Balance the following equations: (m) KOH + H3PO4 ⟶ K3PO4 + H2O (n) CH4 +Br2 ⟶CBr4 +HBr
A:
Q: In the equation: KMnO4 + KBr + H2SO4 → MnSO4 + Br2 + K2SO4 + H2O, if balanced, there would be 10…
A:
Q: Na2CO3(aq) + CaCl2⋅2H2O → CaCO3(s) + 2NaCl(aq) + 2H2O(aq) How many moles of pure CaCl2 are present…
A: To calculate the number of moles: Moles of CaCl2 is given as…
Q: KO2(s) + H2O(l) → KOH(aq)+ O2 (g) + H2O2 (aq)
A: BALENCING EQUATION : 2 KO2 (S) + 2H2 O (l)-------> 2KOH(aq) + O2 (g) + H2O2(aq)
Q: Be sure that all of the chemical equations are properly balanced. a.) COCO3 can be produced…
A: In this question, some equations of reactions are given and mass of specified product is to be…
Q: ON: Balance the following chemical equations, making sure to apply the principle of the Law of…
A:
Q: CHM130LL Take Home Quiz Week 13: Stoichiometry Each correct answer scores the points shown in…
A: [1.] Molar mass of G2ER4 = ( 2 × 20.00 + 15.00 + 4 × 30.00 ) g/mol = 175.00 g/mol Mass% of R =…
Q: Consider the following unbalanced chemical equation: MgS (s) + O2 (g)→ MgO (aq) + SO2 (g) What will…
A: We have to determine the coefficient of O2 in the balanced chemical reaction.
Q: Balance the equation: a ((CH2)30 (CH2O)76 (NH3)16 (H3PO4) ) + b HNO3 = c CO2 + d N2 + e H3PO4 + f…
A: Balanced Equation 5(CH2)30(CH2O)76(NH3)16(H3PO4) + 532HNO3 → 5H3PO4 + 306N2 + 916H2O + 530CO2
Q: Balance each of the following equations: 1. P + 02 -> P2 05 2. NaNO2 -> NaNO2 + O2 3. C8 H18 +…
A: P + 02 -> P2 05 Since oxygen on RHS is 5 and LHS is 2 so in order to have even number we…
Q: When mixed in the presence of water, the elements Zn and I2 react to produce the compound zinc…
A: The balanced chemical reaction is given as : Zn (s) + I2 (s) → ZnI2 (aq)
Q: This equation models how pure silver (Ag) is extracted from ores of AgK(CN)2 (potassium…
A: We have to calculate how many atoms of pure Zn are need in the production process to create a pure…
Q: 4. Balancing a chemical equation involves A. adjusting the subscripts B. adjusting both subscripts…
A: In a chemical reaction, two or more chemical species combined to form another chemical species. The…
Q: Balance the equation: Ni (s) + HCl (aq) → Ni2Cl2 (aq) + H2 (g) How many moles of nickel will react…
A:
Q: equations. (a) KOH + H3PO4 à K3PO4 + H2O (b) Al + H2SO4 à Al2(SO4)3 + H2 (c) Cu + HNO3 à Cu(NO3)2…
A: Balance Chemical equation means no of atoms should be equaal in both side reactant and product
Q: Automotive air bags inflate when sodium azide decomposes explosively to its constituent elements:…
A:
Q: Balance the reaction shown below: KNO3+Ca3(PO4)2→K3PO4+Ca(NO3)2KNO3+Ca3(PO4)2→K3PO4+Ca(NO3)2…
A: Recall the unbalanced given reaction KNO3 + Ca3PO42 → K3PO4 + CaNO32 Here,0.355 grams of KNO3 is…
Q: Balance each of the following chemical reaction equations. Any "blanks" will be considered to be the…
A: A balanced chemical reaction occurs when the number of the atoms involved in the reactants side is…
Q: 1. (3 points) Copper (II) sulfate has been used extensively as a fungicide and herbicide. Copper…
A: Given values- Weight of CuO = 2.49gm Weight of H2SO4=5.05gm Molar mass of CuO = 79.54gm/mole Molar…
Q: 15.3 grams of Lithium is dropped into a solution containing excess copper (II) phosphate. What is…
A: The given balanced chemical reaction is 6 Li + Cu3(PO4)2 → 2 Li3PO4 + 3 Cu From the balanced…
Q: In a car airbag, sodium azide (NaN3) decomposes to form sodium metal and nitrogen gas (reaction1).…
A: Solution : Decomposition reactions are one of the types of reactions in which a reactant broke…
Q: 2) NazSO4 + BaCl2 -> BaSO4 + 2NACI You mix two moles of Na;SO, with one mole BaCl2 a. How many moles…
A: Limiting reactant: The reactant which is completely consumed. Excess reactant: Reactant which…
Q: 1. Balance the following equations: a) NazSO4laq) + Pb(C2H3O2)2(aq) → NaC2H3O2(aq) + PbSO4s) R RAN -…
A: Since you asked multiple questions so as per Q&A guidelines of portal I solve first question…
Q: Balance the equation: As2S3 + HNO3 → H3AsO4 + H2SO4 +NO2 + H2O
A: Given reaction is As2S3 + HNO3 → H3AsO4 + H2SO4 +NO2 + H2O Suppose balance equation is As2S3 + (a)…
Q: 1. Change the following word equations to chemical equations and then balance it. Write the…
A:
Q: Balance the following chemical reactions. 4. K + Br2 ->KBr 5. C8H18 + O2 -> CO2 + H2O 6. Sb + I2 ->…
A:
Q: Balance the following equation - If a coefficient is a one (1) PUT IT IN even though that would be…
A: Balance equation : The Reaction in which Number of atom in reactant side is equal to number of atom…
Q: 1. From the given reaction: Ca(No3)2 + Na3PO4 → Ca3(PO4)2 + NaNO3 a. How many grams of sodium…
A: Please note- As per our company guidelines we are supposed to answer only one question. Kindly…
Q: 8. CaC: H:O Ca(OH): C:H2 9. Mg,N: H:0 _NH) Mg(OH): 10. CaCO, HCI -_CaCl; +cO:
A: To balance an equation, the atoms of all the elements on both sides of the equation must be equal.…
Q: What is the sum of the coefficients of the products in the properly balanced chemical reaction?…
A: A balanced chemical equation states that the number of elements in the reactant side is equal to the…
Q: Part A Urea (CH4N2O) is a common fertilizer that can be synthesized by the reaction of ammonia (NH3)…
A: Answer: NH3 is the chemical formula of the limiting reactant in the reaction.
Q: P2O5? 5. BALANCE the following equation: FeCl3 + AgN03 --------> Fe(NO3)3 + AgCl a) How many grams…
A: given mass of FeCl3 = 75 g mass of Fe(NO3)3 = 1.25 g FeCl3 + AgNO3 → AgCl + Fe(NO3)3
Q: When the following equation is properly balanced using the smallest integer coefficients, what is…
A: Given unbalanced equation: Ca3(PO4)2 (s) + SiO2 (s) + C (s) → P4 (s) + CO (g) + CaSiO3 (s). We have…
Q: When the following equation is balanced, the coefficients are _______. ___Na3PO4(aq) + ___Ni(NO3)2…
A: Balancing equation is to make count of each atom equal on both side. by adding coefficients. The…
Q: Balance each of the following chemical equations. 1. CO2(g)+CaSiO3(s)+H2O(l)→SiO2(s)+Ca(HCO3)2(aq)…
A:
Q: In the balanced chemical equation below, how many grams of lithium carbonate are produced from 0.87…
A:
Q: Balance each of the following chemical reaction equations. Any "blanks" will be considered to be the…
A: Balanced chemical equation is equation that contains same number of atoms for each element that is…
Q: Balance the following chemical equations . IBr + NH3 → NI3 + NH4Br KrF2 + H2O…
A:
Q: 3.12. Balance the following equations. (a) CaC2 (s) + H2O(t) → Ca(OH), (s) + C2H2 (g) (b) (NH,), Cr2…
A: While balancing the reaction, number of atom in reactant should be equal to number of that atom in…
Q: of water d. 2 moles of hydrogen reacte with 1 mole of oxygen to form 2 moles of water 15. The…
A:
Q: In the balanced chemical equation below, how many moles of sodium fluoride are needed to produce…
A:
Q: Balance the following equation: Na2SiO3 (aq) + HF (aq) → H2SiF6 (aq) + NaF(aq) + H2O(l)
A: Balancing the above equation through hit and trial method.
Q: Balance these equations: ____ P4 + ____ H2 => ____ PH3 ____ SiCl4 + ____ Mg => ____ Si + ____…
A: Balance these equations:
Q: Elemental phosphorus is produced from calcium phosphate in the following reaction. What is the…
A: The given reaction is a redox reaction, these are the types of reaction in which oxidation as well…
Q: The coefficients in the balanced equation below are respectfully: _____HNO2(aq) + _____Tl+(aq)…
A:
Step by step
Solved in 2 steps with 1 images

- Mass of Na2CO3.H2O (g) = 2.12g (g) Mass of the CaCl2.2H2O (g) = 1.98g Mass of the top funnel + filter paper (g) = 15.85g Mass of top funnel + filter paper + CaCO3 collected (g) = 17.81g CaCl2 + Na2CO3 ==== CaCo3 + 2NaCl Theoretical yield in moles and grams? Moles of reagent in excess left unreacted? Mass of precipitate? Experimental yield? Percent yield?1. Balance the following equations:a) ____ Na2SO4(aq) + ____ Pb(C2H3O2)2(aq) → ____ NaC2H3O2(aq) + ____ PbSO4(s) i. How many moles of Pb(C2H3O2)2 are required to react with 1.67 moles Na2SO4? Use dimensional analysis.In batteries factory, the manager asked the technician to prepare a batch of battery acid with a concentration of 23.6% (wt %) as follows Raw materials (streams) Stream 1: Old weak battery acid (H2SO4) solution contains 16.9% (wt %) of H2SO4 (the rest is pure water) Stream 2:250 kg of 65.2 % H2SO4 How many kilograms of old weak battery acid (H2SO4) are needed to prepare the desired ?battery acid (23.6%) .a o 3313.43 Kg bo 1755.25 Kg .CO 1552.24 Kg .do 3875.14 Kg
- 1. Balance the following chemical equation: Fe2O3 + CO ------> Fe + CO2, answers are listed in order - 1st coefficient, 2nd coeficient, etc. and so on. (1st one is in front of Fe2O3, 2nd one in front of CO, etc. and so on) Group of answer choices a) 1,3,3,2 b) 2,3,2,3 c) 1,3,2,3 d) 1,2,2,2Balance the following equation in standard form and determine the sum of the coefficients. LiAlH4(s) + AlCl3(s) --> AlH3(s) + LiCl(s) Group of answer choices 9 11 10 12 8balance: KMnO4(aq) + Na2SO3(aq) + KOH → MnO2 + Na2SO4(aq) ; the coefficient for Na2SO3 is: (hint: keep MnO2 intact) A. 2 B. 1 C. 3
- 9.) Which set of coefficients balances the following equation? Cu(NO3)2 (aq) + (NH4)2S (aq) --> CuS (s) + NH4NO3 (aq) A) 1, 1, 1, 1 B) 1, 1, 1, 2 C) 2, 2, 2, 4 D) none of the aboveMass of Na2CO3.H2O (g) = 2.12g (g) Mass of the CaCl2.2H2O (g) = 1.98g Mass of the top funnel + filter paper (g) = 15.85g Mass of top funnel + filter paper + CaCO3 collected (g) = 17.81g CaCl2 + Na2CO3 ==== CaCo3 + 2NaCl Moles Na2CO3 reacting? Moles CaCO3 reacting? Moles CaCO3 produced by the LR? Theoretical yield in moles and grams? Reagent in excess? Moles of reagent in excess left unreacted? Mass of precipitate? Experimental yield? Percent yield?The balanced reaction of oxalic acid with potassium dichromate in an acidic solution is: 3H2C2O4(aq) + K2Cr2O7(aq) + 5H2SO4(aq) --> 2KHSO4(aq) Cr2(SO4)3(aq) 6CO2(g) 7H2O(I) In an experiment, 2.7000g of H2C2O4, 2.8500g of K2Cr2O7, and 3.5000g of H2SO4 are combined and 1.7500g of KHSO4 and 2.500g of Cr2(SO4)3 are recovered. 1) Find the limiting reagent. 2) Find the theoretical yield of both KHSO4 and Cr2(SO4)3 in grams. 3) Find the amounts of excess reagent left over in grams. 4) Find the Percent Yield of both KHSO4 and Cr2(SO4)3.
- mass of cuso4: 7.00g. mass of fe: 2.00g. mass of filter paper: 1.50g. mass of cu solid: 2.16g. mass of dried filter paper and cu solid: 3.96g. limiting reagent: cuso4. calculate the moles of cu(s) formed along with the moles of limiting reagent used and ratio of moles of cu(s) formed/moles of limiting reagent.Adipic acid, H2C6H8O4, is used to produce nylon. It is made commercially by a controlled reaction between cyclohexane ( C6H12 ) and O2:. (Only the 4th sub part (d) is needed to be answered). 2 C6H12 (l) + 5 O2 (g) → 2 H2C6H8O4 (l) + 2 H2O (g) (Given: Atomic Wts (g/mol): C= 12.01 ; H=1.01 ; O=16.00 ) If 25.0 g of cyclohexane is reacted with 20.0 g of O2, a. Identify the limiting reactant and excess reactant? b. How much in grams of the excess reactant will be left after the reaction? c. What is the theoretical yield in terms of the product adipic acid? d. If the actual yield is 33.5 g, what is the % yield of adipic acid?Please view the video* below and answer the following questions.https://www.youtube.com/watch?v=1ocQhkHw_MM1. Two reactions were demonstrated in the video above. Write the balanced equations for both reactions below.a.b.2. What catalyst was used in the decomposition of hydrogen peroxide?3. Which safety rule is broken during the demonstrations shown in this video?4. View the video and complete the table below.https://www.youtube.com/watch?v=_G7sNq7R29w Observations Balanced Reaction Equation 5. View the video and complete the table below.https://www.youtube.com/watch?v=yLsjsy_Dqd0 Observations Balanced Reaction Equation

