3. Below are two other organic dyes that both produce a yellow color when dissolved. Which of the solvents (water, ethyl acetate, ethanol, dichloromethane, HCl) (select one solvent for each dye (dyes include disperse red 13 and methylene blue) could be used to dissolve samples of each of the dyes (individual samples, not a mixture)? HN- Radiant Yellow OH poro Alizarine Yellow 0 O Na
3. Below are two other organic dyes that both produce a yellow color when dissolved. Which of the solvents (water, ethyl acetate, ethanol, dichloromethane, HCl) (select one solvent for each dye (dyes include disperse red 13 and methylene blue) could be used to dissolve samples of each of the dyes (individual samples, not a mixture)? HN- Radiant Yellow OH poro Alizarine Yellow 0 O Na
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter5: Thermochemistry
Section: Chapter Questions
Problem 44E: Although the gas used in an oxyacetylene torch (Figure 5.7) is essentially pure acetylene, the heat...
Related questions
Question
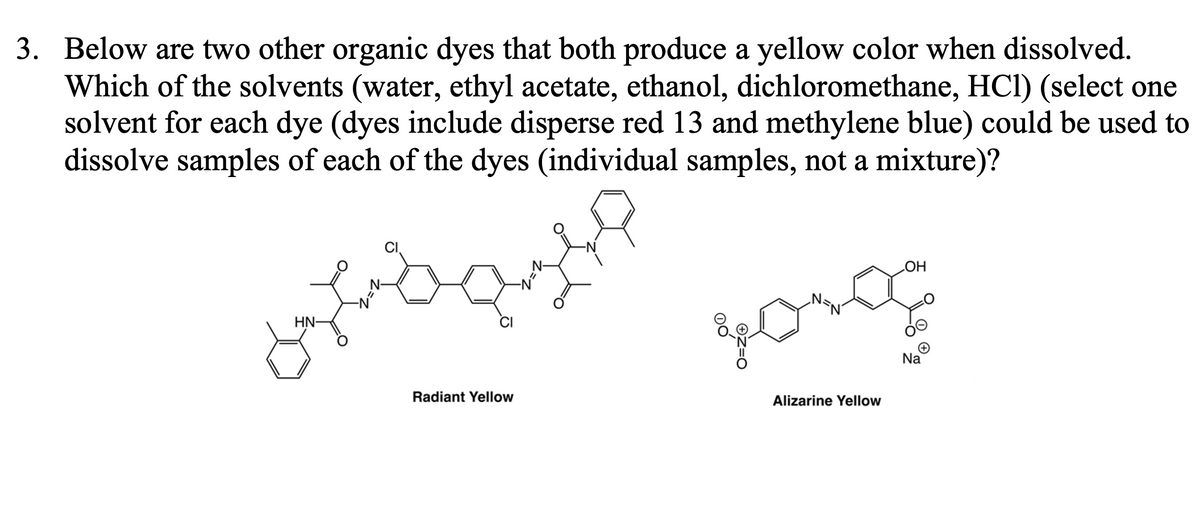
Transcribed Image Text:3. Below are two other organic dyes that both produce a yellow color when dissolved.
Which of the solvents (water, ethyl acetate, ethanol, dichloromethane, HC1) (select one
solvent for each dye (dyes include disperse red 13 and methylene blue) could be used to
dissolve samples of each of the dyes (individual samples, not a mixture)?
HN-
Radiant Yellow
=1
долод
Alizarine Yellow
OH
Na
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning