3. Find the coefficient of thermal expansion a and the isothermal compressibility k from each of the following p-v-T relations: RT (a) Ideal gas equation, p = RT (b) Dieterici equation, p = e a/RTU %3D RT (c) Saha-Bose equation, p: e-a/RTV In 2b - 2b In the above equations R is the gas constant and a and b are specific constants. Verify that for large values of T and v all the expressions for a and K go over into the corresponding expressions for an ideal gas.
3. Find the coefficient of thermal expansion a and the isothermal compressibility k from each of the following p-v-T relations: RT (a) Ideal gas equation, p = RT (b) Dieterici equation, p = e a/RTU %3D RT (c) Saha-Bose equation, p: e-a/RTV In 2b - 2b In the above equations R is the gas constant and a and b are specific constants. Verify that for large values of T and v all the expressions for a and K go over into the corresponding expressions for an ideal gas.
Principles of Physics: A Calculus-Based Text
5th Edition
ISBN:9781133104261
Author:Raymond A. Serway, John W. Jewett
Publisher:Raymond A. Serway, John W. Jewett
Chapter16: Temperature And The Kinetic Theory Of Gases
Section: Chapter Questions
Problem 66P
Related questions
Question
Kindly i need solution for this question asap with clear font
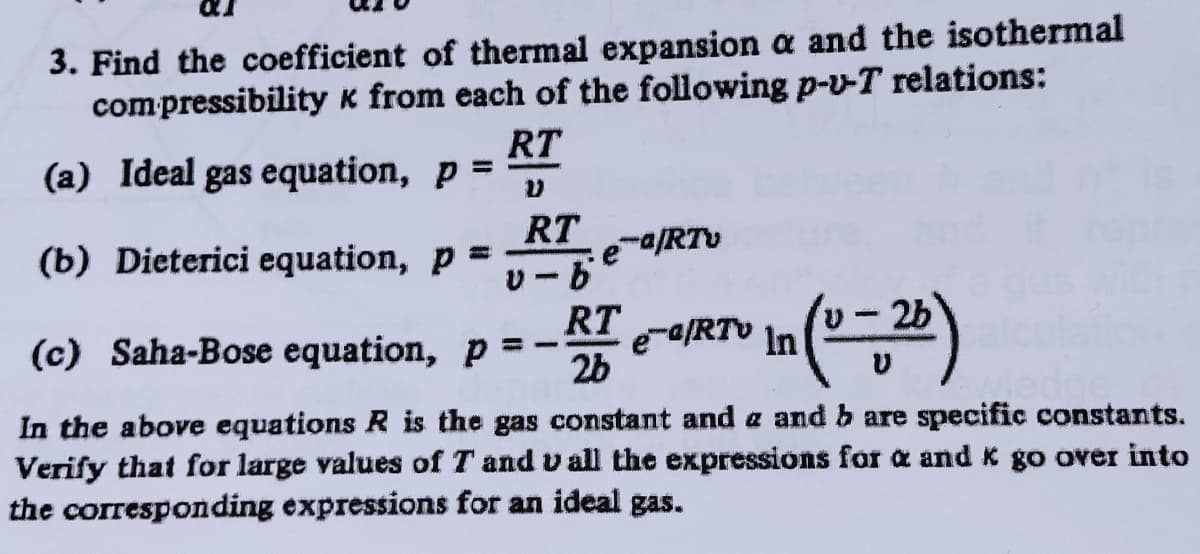
Transcribed Image Text:3. Find the coefficient of thermal expansion a and the isothermal
compressibility k from each of the following p-v-T relations:
RT
(a) Ideal gas equation, p =
%3D
RT
e-/RTv
(b) Dieterici equation, p =
U-2b
RT
e-aRTV In
2b
|3
(c) Saha-Bose equation, p
In the above equations R is the gas constant and a and b are specific constants.
Verify that for large values of T and v all the expressions for a and K go over into
the corresponding expressions for an ideal gas.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers with Modern …
Physics
ISBN:
9781337553292
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers
Physics
ISBN:
9781337553278
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers with Modern …
Physics
ISBN:
9781337553292
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers
Physics
ISBN:
9781337553278
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers, Technology …
Physics
ISBN:
9781305116399
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College

College Physics
Physics
ISBN:
9781285737027
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning