3. Standard white vinegar you can buy in the grocery store is 5% concentration. That means 5% of the liquid vinegar is acetic acid and 95% of the solution is water. In a hardware store, you can buy indu strength vinegar, which is 30% concentration. This means that 30% of the vinegar is acetic acid, and the remaining 70% is water. Samuel does another experiment, this time with 5% vinegar and 30% vinegar. He sets up two science fair volcanoes (in no particular order), each with the same temperature, mass of baking soda and volur vinegar. But one volcano uses 5% vinegar and the other volcano uses 30% vinegar. He measures the volume of gas production for thefirst minute of each reaction, and he records the data below. Volcano # 1 Volume of gas produced (mt) vs. Time (s) for Volcano #n Volume of gas produced (ml) Time (s) 50 10 20 25 38 40 30 40 46 30 Volume of gas produced (ml.) 50 20 52 53 50 10 60 40 60 Time (s) Volcano # 2 Volume of gas produced (mL) vs. Time (s) for Vocano 2 Volume of gas produced (ml.) Time 50 45 40 35 30 25 20 10 20 30 10 19 26 33 Volume of gas produced int) 40 30 10 60 45 20 80 Tm e (u) peonpoud sed jo ounoa peonpead sed o un
3. Standard white vinegar you can buy in the grocery store is 5% concentration. That means 5% of the liquid vinegar is acetic acid and 95% of the solution is water. In a hardware store, you can buy indu strength vinegar, which is 30% concentration. This means that 30% of the vinegar is acetic acid, and the remaining 70% is water. Samuel does another experiment, this time with 5% vinegar and 30% vinegar. He sets up two science fair volcanoes (in no particular order), each with the same temperature, mass of baking soda and volur vinegar. But one volcano uses 5% vinegar and the other volcano uses 30% vinegar. He measures the volume of gas production for thefirst minute of each reaction, and he records the data below. Volcano # 1 Volume of gas produced (mt) vs. Time (s) for Volcano #n Volume of gas produced (ml) Time (s) 50 10 20 25 38 40 30 40 46 30 Volume of gas produced (ml.) 50 20 52 53 50 10 60 40 60 Time (s) Volcano # 2 Volume of gas produced (mL) vs. Time (s) for Vocano 2 Volume of gas produced (ml.) Time 50 45 40 35 30 25 20 10 20 30 10 19 26 33 Volume of gas produced int) 40 30 10 60 45 20 80 Tm e (u) peonpoud sed jo ounoa peonpead sed o un
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter4: Chemical Reactions In Solution
Section: Chapter Questions
Problem 4.9QE: Addition of water to concentrated sulfuric acid is dangerous because it generates enough heat to...
Related questions
Question
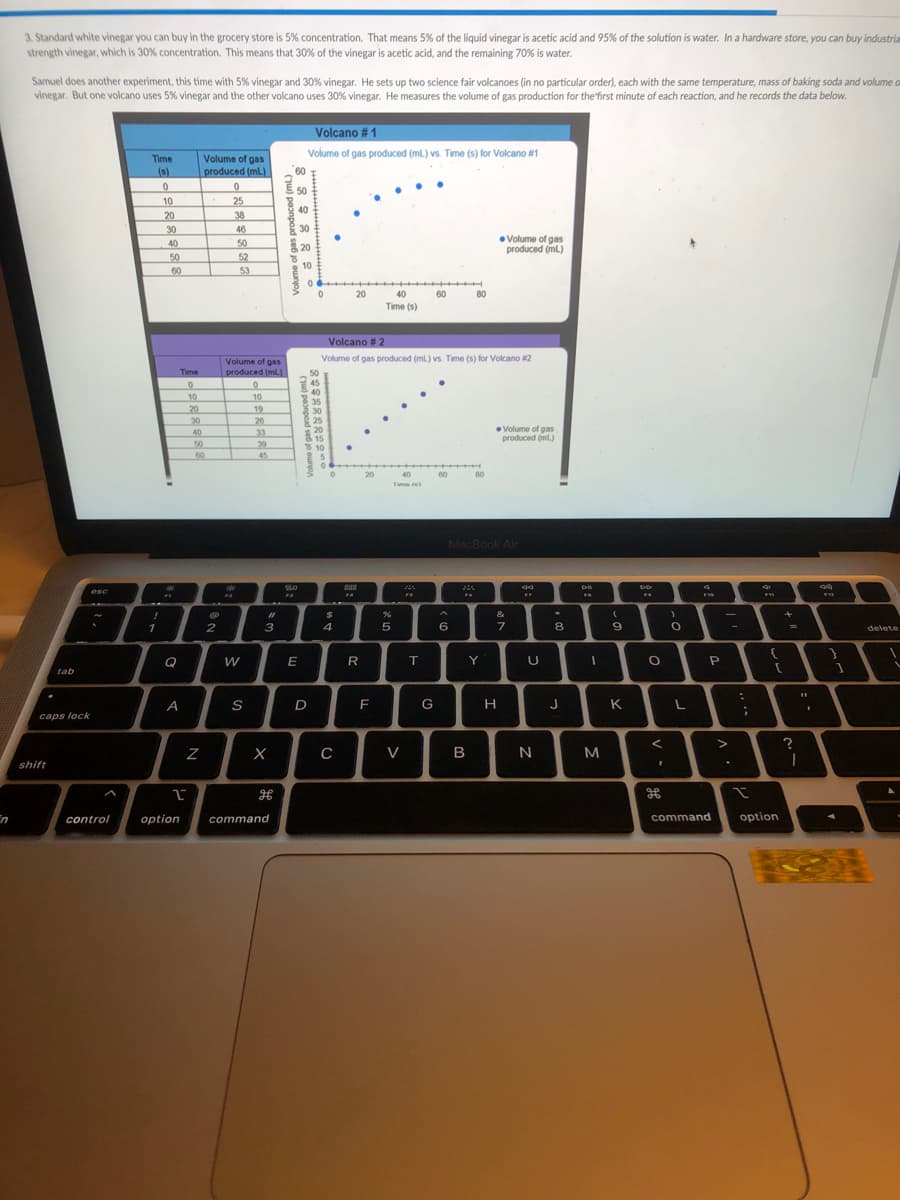
Transcribed Image Text:3. Standard white vinegar you can buy in the grocery store is 5% concentration. That means 5% of the liquid vinegar is acetic acid and 95% of the solution is water. In a hardware store, you can buy industria
strength vinegar, which is 30% concentration. This means that 30% of the vinegar is acetic acid, and the remaining 70% is water.
Samuel does another experiment, this time with 5% vinegar and 30% vinegar. He sets up two science fair volcanoes (in no particular order), each with the same temperature, mass of baking soda and volume o
vinegar. But one volcano uses 5% vinegar and the other volcano uses 30% vinegar. He measures the volume of gas production for the'first minute of each reaction, and he records the data below.
Volcano # 1
Volume of gas produced (mL.) vs. Time (s) for Volcano #1
Time
Volume of gas
(s)
produced (ml)
60
E 50
10
20
30
25
8 40
38
46
30
20
Volume of gas
produced (ml)
40
50
50
60
52
of
10
53
20
40
60
80
Time (s)
Volcano # 2
Volume of gas
Volume of gas produced (ml.) vs. Time (s) for Volcano 2
Time
produced (mL)
50
45
40
35
10
20
30
40
10
19
30
26
e 25
Volume of gas
produced (ml.)
33
20
50
39
8 10
60
45
20
40
60
80
Time te
MacBook Air
SLO
esc
%23
&
1
3
4
6.
8.
delete
Q
E
R
Y
U
tab
A
S
D
F
caps lock
V
B
M
shift
control
option
command
command
option
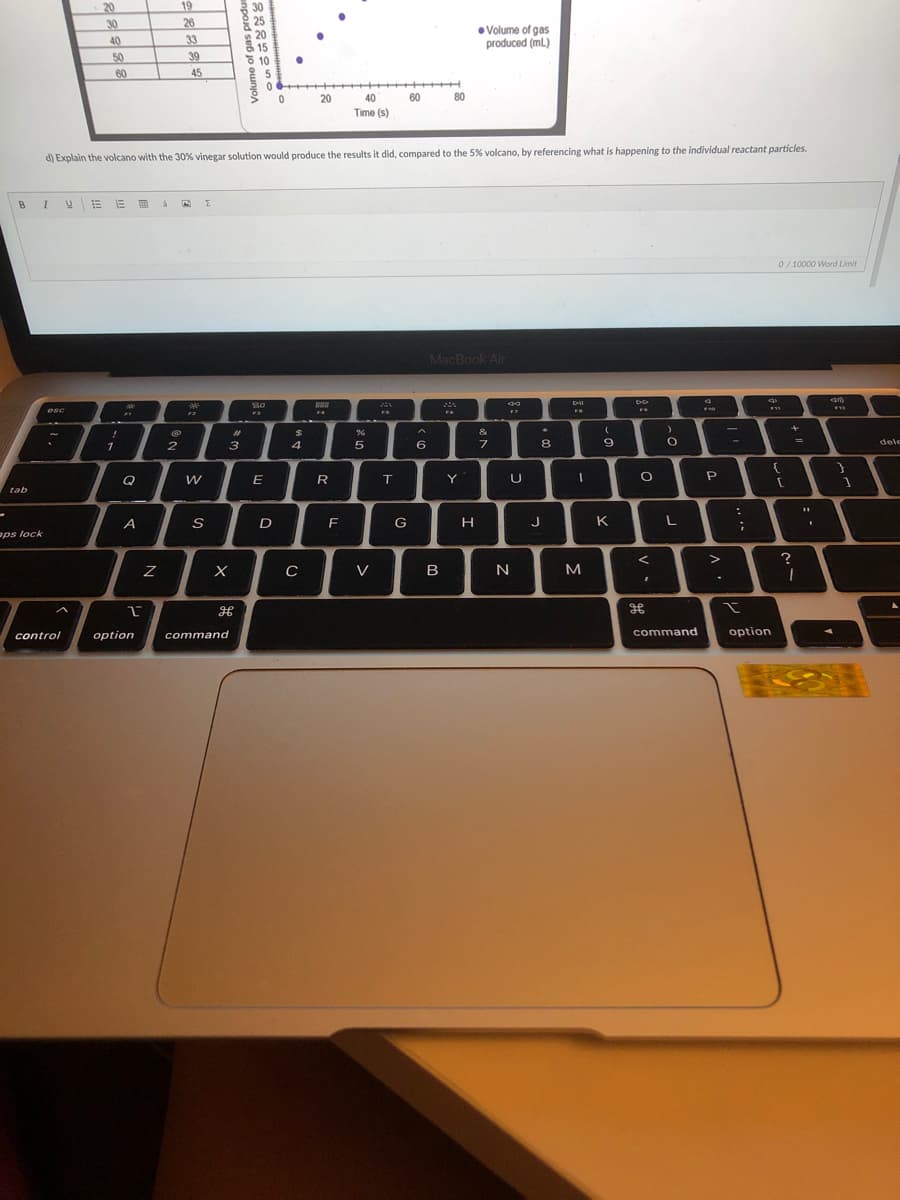
Transcribed Image Text:20
30
19
26
• Volume of gas
produced (mL)
40
33
39
45
50
60
20
40
60
80
Time (s)
d) Explain the volcano with the 30% vinegar solution would produce the results it did, compared to the 5% volcano, by referencing what is happening to the individual reactant particles.
B
0/10000 Word Limit
MacBook Air
888
F
esc
F7
FA
%23
%24
&
8.
6.
%3D
dele
2
3
4
5
6.
Q
Y
U
P
tab
D
F
G
H.
J
K
ops lock
V
B
option
command
command
option
control
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning
