Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter2: Chemical Compounds
Section: Chapter Questions
Problem 143QRT: The present average concentration (mass percent) of magnesium ions in seawater is 0.13%. A chemistry...
Related questions
Question
Answer for number 3
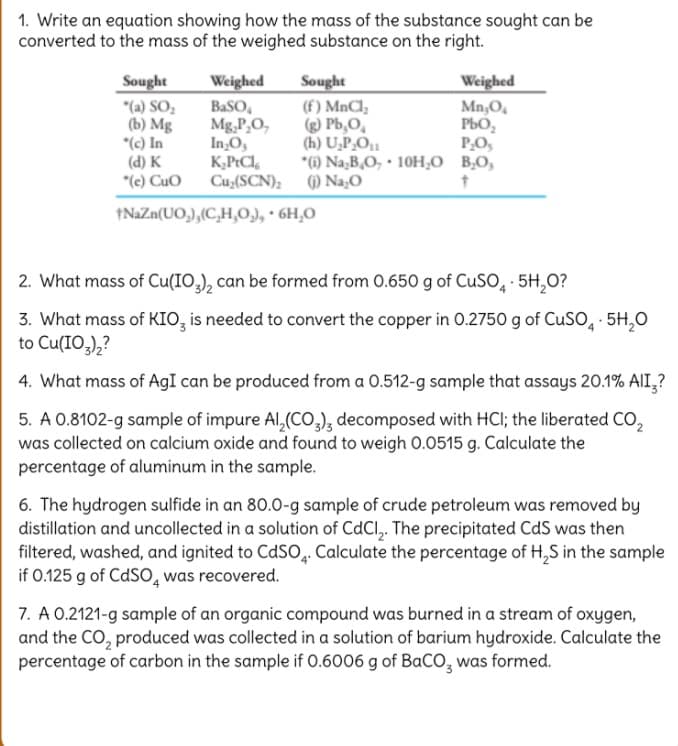
Transcribed Image Text:1. Write an equation showing how the mass of the substance sought can be
converted to the mass of the weighed substance on the right.
Sought
"(a) SO,
(b) Mg
*(c) In
(d) K
"(e) Cuo
Sought
(f) MnCl,
) Pb,O,
(h) U,P,O1
*6) Na,B,O, · 10H,O B,O,
Weighed
Weighed
BaSO,
Mg,P,O,
In,O,
K,PtCl,
Cu,(SCN); () Na,O
Mn,O,
PbO,
P,O,
1NAZN(UO,),(C,H,0), · 6H,0
2. What mass of Cu(IO,), can be formed from 0.650 g of CUSO, · 5H,0?
3. What mass of KIO, is needed to convert the copper in 0.2750 g of CUSO, · 5H,0
to Cu(IO,),?
4. What mass of AgI can be produced from a 0.512-g sample that assays 20.1% AlI,?
5. A 0.8102-g sample of impure AI,(CO,), decomposed with HCI; the liberated CO,
was collected on calcium oxide and found to weigh 0.0515 g. Calculate the
percentage of aluminum in the sample.
6. The hydrogen sulfide in an 80.0-g sample of crude petroleum was removed by
distillation and uncollected in a solution of CdCl,. The precipitated CdS was then
filtered, washed, and ignited to CdSO. Calculate the percentage of H,S in the sample
if 0.125 g of CdSO, was recovered.
7. A 0.2121-g sample of an organic compound was burned in a stream of oxygen,
and the CO, produced was collected ina solution of barium hydroxide. Calculate the
percentage of carbon in the sample if 0.6006 g of BaCO, was formed.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co