3.12 This problem has several parts: a. If a portable electronic device draws 1 A current at a voltage of 2.5 V, what is the power requirement for the device? b. You have designed a fuel cell that delivers 1 A at 0.5 V. How many of your fuel cells are required to supply the above portable electronic device with its necessary voltage and current requirements? c. You would like the portable electronic device to have an operating lifetime of 100 h. Assuming 100% fuel utilization, what is the minimum amount of H, fuel (in grams) required?
3.12 This problem has several parts: a. If a portable electronic device draws 1 A current at a voltage of 2.5 V, what is the power requirement for the device? b. You have designed a fuel cell that delivers 1 A at 0.5 V. How many of your fuel cells are required to supply the above portable electronic device with its necessary voltage and current requirements? c. You would like the portable electronic device to have an operating lifetime of 100 h. Assuming 100% fuel utilization, what is the minimum amount of H, fuel (in grams) required?
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Chapter1: Introduction
Section: Chapter Questions
Problem 1.1P
Related questions
Question
Please i need help .
solving a,b,c,d,e
the second picture is answers key for them
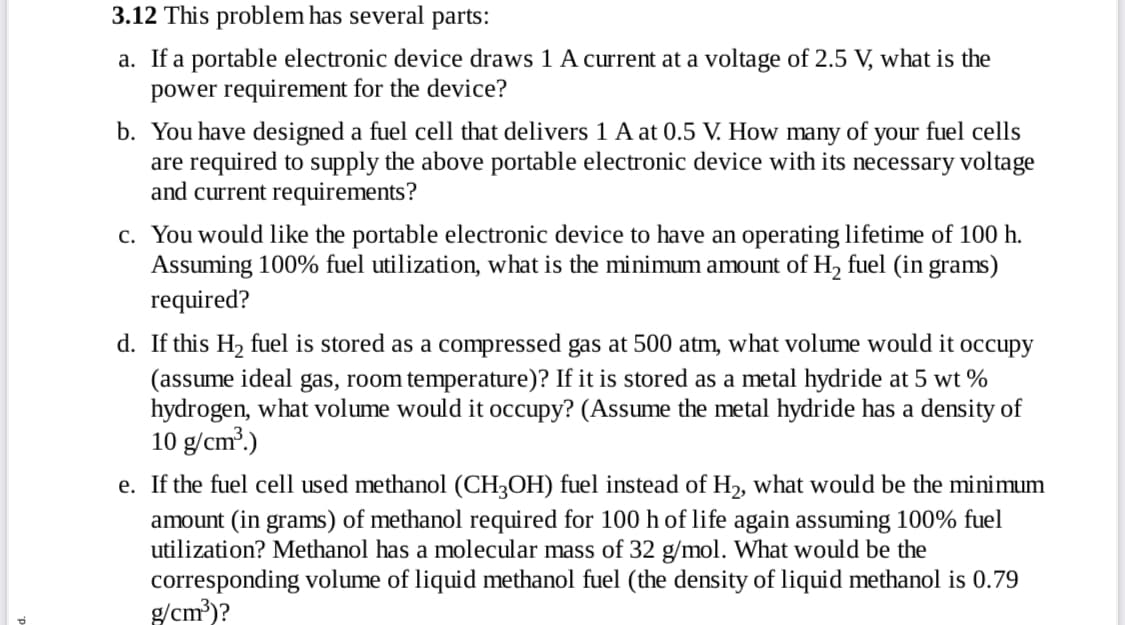
Transcribed Image Text:3.12 This problem has several
parts:
a. If a portable electronic device draws 1 A current at a voltage of 2.5 V, what is the
power requirement for the device?
b. You have designed a fuel cell that delivers 1 A at 0.5 V. How many of your fuel cells
are required to supply the above portable electronic device with its necessary voltage
and current requirements?
c. You would like the portable electronic device to have an operating lifetime of 100 h.
Assuming 100% fuel utilization, what is the minimum amount of H, fuel (in grams)
required?
d. If this H2 fuel is stored as a compressed gas at 500 atm, what volume would it occupy
(assume ideal gas, room temperature)? If it is stored as a metal hydride at 5 wt %
hydrogen, what volume would it occupy? (Assume the metal hydride has a density of
10 g/cm?.)
e. If the fuel cell used methanol (CH3OH) fuel instead of H2, what would be the minimum
amount (in grams) of methanol required for 100 h of life again assuming 100% fuel
utilization? Methanol has a molecular mass of 32 g/mol. What would be the
corresponding volume of liquid methanol fuel (the density of liquid methanol is 0.79
g/cm?)?
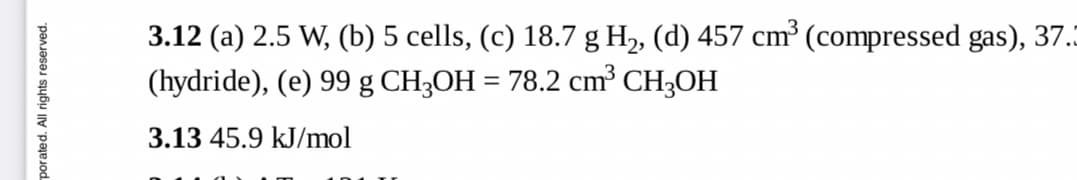
Transcribed Image Text:3.12 (a) 2.5 W, (b) 5 cells, (c) 18.7 g H2, (d) 457 cm³ (compressed gas), 37.
(hydride), (e) 99 g CH3OH = 78.2 cm³ CH3OH
3.13 45.9 kJ/mol
porated. All rights reserved.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 7 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemical-engineering and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:
9781285061238
Author:
Lokensgard, Erik
Publisher:
Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:
9780072848236
Author:
Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:
McGraw-Hill Companies, The