*** 32. A double-chambered container contains one mole of helium in one of its 1000 cm³ Pull to volume chambers. The container is well-insulated, and of low specific heat, so that no appre- ciable heat is added to the gas during the process we describe. The gas is initially at a tempera- ture of 300 K and a pressure of 1 atmosphere. The partition between the two chambers is then quickly raised, and the gas expands freely to fill the entire container. Whenever a monatomic gas like helium doubles its volume adiabatically like this, the pressure in the gas will drop to 0.315 of what it was before (for reasons that we did not explain in this chapter), so the final pressure of the expanded gas will be 0.315 atmospheres. remove 1000 cm³ 1000 cm³ a) What is the temperature of the gas after the expansion? b) What is the change in the internal energy of the gas? c) How much heat is added to the gas? [Hint: Maybe read the problem again.] d) How much work is done by the gas as it expands? [Hint: The pressure is not constant, so you cannot use the formula W = pAV.]
*** 32. A double-chambered container contains one mole of helium in one of its 1000 cm³ Pull to volume chambers. The container is well-insulated, and of low specific heat, so that no appre- ciable heat is added to the gas during the process we describe. The gas is initially at a tempera- ture of 300 K and a pressure of 1 atmosphere. The partition between the two chambers is then quickly raised, and the gas expands freely to fill the entire container. Whenever a monatomic gas like helium doubles its volume adiabatically like this, the pressure in the gas will drop to 0.315 of what it was before (for reasons that we did not explain in this chapter), so the final pressure of the expanded gas will be 0.315 atmospheres. remove 1000 cm³ 1000 cm³ a) What is the temperature of the gas after the expansion? b) What is the change in the internal energy of the gas? c) How much heat is added to the gas? [Hint: Maybe read the problem again.] d) How much work is done by the gas as it expands? [Hint: The pressure is not constant, so you cannot use the formula W = pAV.]
Principles of Heat Transfer (Activate Learning with these NEW titles from Engineering!)
8th Edition
ISBN:9781305387102
Author:Kreith, Frank; Manglik, Raj M.
Publisher:Kreith, Frank; Manglik, Raj M.
Chapter8: Natural Convection
Section: Chapter Questions
Problem 8.34P
Related questions
Question
![*** 32. A double-chambered container contains one mole of helium in one of its 1000 cm³
Pull to
volume chambers. The container is well-insulated, and of low specific heat, so that no appre-
remove
ciable heat is added to the gas during the process we describe. The gas is initially at a tempera-
ture of 300 K and a pressure of 1 atmosphere. The partition between the two chambers is then
quickly raised, and the gas expands freely to fill the entire container. Whenever a monatomic
gas like helium doubles its volume adiabatically like this, the pressure in the gas will drop to
0.315 of what it was before (for reasons that we did not explain in this chapter), so the final
1000 cm3
1000 cm³
pressure of the expanded gas will be 0.315 atmospheres.
a) What is the temperature of the gas after the expansion?
b) What is the change in the internal energy of the gas?
c) How much heat is added to the gas? [Hint: Maybe read the problem again.]
d) How much work is done by the gas as it expands? [Hint: The pressure is not constant,
so you cannot use the formula W = pAV.]](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F776b81b2-5984-443d-93b2-d351507602c0%2Fed54f85d-b0cd-4543-8a98-ee638ddc379d%2Fgwir45_processed.png&w=3840&q=75)
Transcribed Image Text:*** 32. A double-chambered container contains one mole of helium in one of its 1000 cm³
Pull to
volume chambers. The container is well-insulated, and of low specific heat, so that no appre-
remove
ciable heat is added to the gas during the process we describe. The gas is initially at a tempera-
ture of 300 K and a pressure of 1 atmosphere. The partition between the two chambers is then
quickly raised, and the gas expands freely to fill the entire container. Whenever a monatomic
gas like helium doubles its volume adiabatically like this, the pressure in the gas will drop to
0.315 of what it was before (for reasons that we did not explain in this chapter), so the final
1000 cm3
1000 cm³
pressure of the expanded gas will be 0.315 atmospheres.
a) What is the temperature of the gas after the expansion?
b) What is the change in the internal energy of the gas?
c) How much heat is added to the gas? [Hint: Maybe read the problem again.]
d) How much work is done by the gas as it expands? [Hint: The pressure is not constant,
so you cannot use the formula W = pAV.]
Expert Solution
Step 1
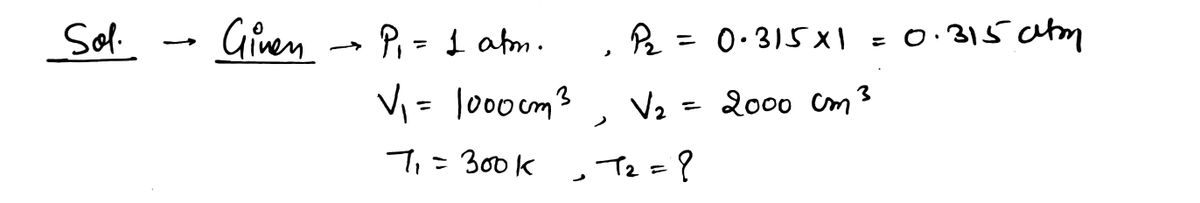
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Heat Transfer (Activate Learning wi…
Mechanical Engineering
ISBN:
9781305387102
Author:
Kreith, Frank; Manglik, Raj M.
Publisher:
Cengage Learning

Principles of Heat Transfer (Activate Learning wi…
Mechanical Engineering
ISBN:
9781305387102
Author:
Kreith, Frank; Manglik, Raj M.
Publisher:
Cengage Learning