32. A gasoline engine takes in 2500 J of the heat and delivers 500J of mechanical work per cycle. The heat is obtained by burning gasoline with heat of combustion L. = 5.0 x 10 J-g. What is the thermal efficiency of this engine? A. 15% B. 20%* C. 30% D. 14% 33
32. A gasoline engine takes in 2500 J of the heat and delivers 500J of mechanical work per cycle. The heat is obtained by burning gasoline with heat of combustion L. = 5.0 x 10 J-g. What is the thermal efficiency of this engine? A. 15% B. 20%* C. 30% D. 14% 33
College Physics
1st Edition
ISBN:9781938168000
Author:Paul Peter Urone, Roger Hinrichs
Publisher:Paul Peter Urone, Roger Hinrichs
Chapter14: Heat And Heat Transfer Methods
Section: Chapter Questions
Problem 53PE: A person inhales and exhales 2.00 L of 37.0C air, evaporating 4.00102g of water from the lungs and...
Related questions
Question

Transcribed Image Text:32. A gasoline engine takes in 2500 J of the heat and delivers 500J of mechanical
work per cycle. The heat is obtained by burning gasoline with heat of
combustion L. = 5.0 x 104 J-g. What is the thermal efficiency of this engine?
A. 15%
B. 20%*
C. 30%
D. 14%
33. A gasoline engine takes in 2500 J of the heat and delivers 500J of mechanical
work per cycle. The heat is obtained by burning gasoline with heat of
combustion Le = 5.0 x 104 J-g. How much heat is discarded in each cycle?
A. -2000 Joules*
B. -1000 Joules
C. -3000 Joules
D. -4000 Joules
34. A gasoline engine takes in 2500 J of the heat and delivers 500J of mechanical
work per cycle. The heat is obtained by burning gasoline with heat of
combustion L. = 5.0 x 104 J-g-1. How much gasoline is burned in each cycle?
A. 0.04 gr
B. 0.02 gr
C. 0.01 gr
D. 0.05 gr*
35. What is the total random Kinetic energy of the molecules in 1 mol of a gas a
temperature of 3OOK?
A. 3741 Joules*
B. 2654 Joules
O. 1056 oules
D. 7123 Uoules
36. A Carnot engine takes 2000J of heat from a reservoir at 5o0 K
and discards some heat to a reservoir at 05OK.HOMmuch works does the engine
do, how much heat is discarded, and what 1s the efficiency?
A. -3600 Joule, 100 Joule,
B. -1400 Joule, 6po Joule, 30$*
does some work
C. -2500 Goule, So CTbule, 904
D.-1000 Joule, 30OWToule, 105
50
37. I the cycle deScribed in prob. 33 run backward as a refrigerator. What is the
perfcrmance coefficient?
A. 9.06
B..93-
C. 3.15
D. 1.5
L88.One kilogram of ice a: °c 15 meited and converted to vater at 0 c. Compute
4ce change in ertropy?
AL200 Joule/-
4/10
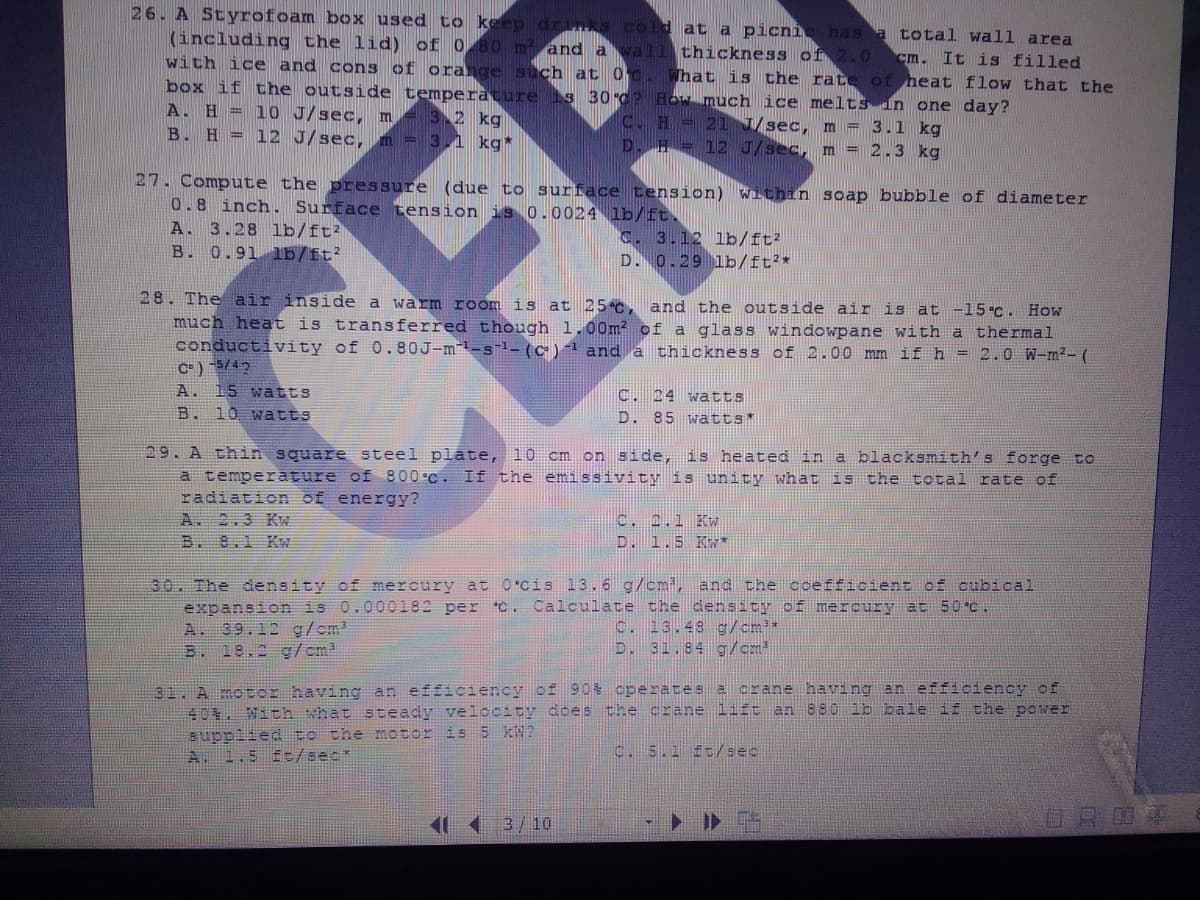
Transcribed Image Text:26. A Styrofoam box used to keep drinks cold at a picní has a total wall area
(including the lid) of 0 80 m and a vall thickness of 2.0 cm. It is filled
with ice and cons of orange such at 0 c. What is the rate of heat flow that the
box if the outside temperature 1s 30d? How much ice melts in one day?
A. H = 10 J/sec, m = 32 kg
B. H = 12 J/sec, m = 3,1 kg*
C. H 21 /sec, m = 3.1 kg
D. H = 12 J/sec, m = 2.3 kg
27. Compute the pressure (due to surface tension) within soap bubble of diameter
0.8 inch. Surface tension is 0.0024 lb/ft.
A. 3.28 lb/ft?
B. 0.91 lb/ft?
C. 3.12 lb/ft?
D. 0.29 lb/ft?*
28. The air inside a warm room is at 25 C, and the outside air is at -15.C. How
much heat is transferred though 1.00m? of a glass windowpane with a thermal
conductivity of 0.80J-m-s-1- (c)1 and a
C= ) 5/42
A. 15 watts
B. 10 watts
thickness of 2.00 mm if h = 2.0 W-m²- (
CER
C. 24 watts
D. 85 wätts*
29. A thin square steel plate, 10 cm on side, is heated in a blacksmith's forge to
a temperature of 800°C. If the emissivity is unity what is the total rate of
radiation of energy?
A. 2.3 KW
B. 8.1 K
C. 2.1 Kw
D. 1.5 K*
30. The density of mercury at O cis 13.6 g/cmi, and the coefficient of cubical
expansion is 0.000182 per C. Calculate the density of mercury
A. 39.12 g/cm?
B. 18.2 g/cm?
at 50°C.
C. 13.48 g/cm*
D. 31.84 g/cm
31 A motor having an efficiency of 90% operates a crane having an efficiency of
40. With what steady velocity does the crane lift an 880 lb bale if the power
suppiied to the motor is 5 KW?
A. 1.5 f:/sec*
c. 5.1 ft/sec
11 1 3/10
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you

College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College

Physics for Scientists and Engineers, Technology …
Physics
ISBN:
9781305116399
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning


College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College

Physics for Scientists and Engineers, Technology …
Physics
ISBN:
9781305116399
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning


College Physics
Physics
ISBN:
9781285737027
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781305952300
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning
