34. Which of the following is the correct equation for the reaction that occurs in this battery? A. Zn(s) + Cr20,2-(aq) + 14 H1+(aq) → Zn²*(aq) + 2 Cr3*(aq) + 7 H2O(t) B. 3 Zn(s) + Cr2072-(aq) + 14 H1+(aq) → 3 Zn2+(aq) + 2 Cr3+(aq) + 7 H2O(£) C. 3 Zn2+(aq) + 2 Cr3+ (aq) + 7 H2O(t) → 3 Zn(s) + Cr2077¯(aq) + 14 H1+(aq) D. 3 Zn(s) + 2 Cr3+ (aq) + 7 H20(e) → 3 Zn2+(aq) + Cr20,7¯(aq) + 14 H1+(aq)
34. Which of the following is the correct equation for the reaction that occurs in this battery? A. Zn(s) + Cr20,2-(aq) + 14 H1+(aq) → Zn²*(aq) + 2 Cr3*(aq) + 7 H2O(t) B. 3 Zn(s) + Cr2072-(aq) + 14 H1+(aq) → 3 Zn2+(aq) + 2 Cr3+(aq) + 7 H2O(£) C. 3 Zn2+(aq) + 2 Cr3+ (aq) + 7 H2O(t) → 3 Zn(s) + Cr2077¯(aq) + 14 H1+(aq) D. 3 Zn(s) + 2 Cr3+ (aq) + 7 H20(e) → 3 Zn2+(aq) + Cr20,7¯(aq) + 14 H1+(aq)
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter17: Electrochemistry
Section: Chapter Questions
Problem 80QAP: A solution containing a metal ion (M2+(aq)) is electrolyzed by a current of 7.8 A. After 15.5...
Related questions
Question
Hi, the picture with the chart is the battery referred to. Thank you so much.
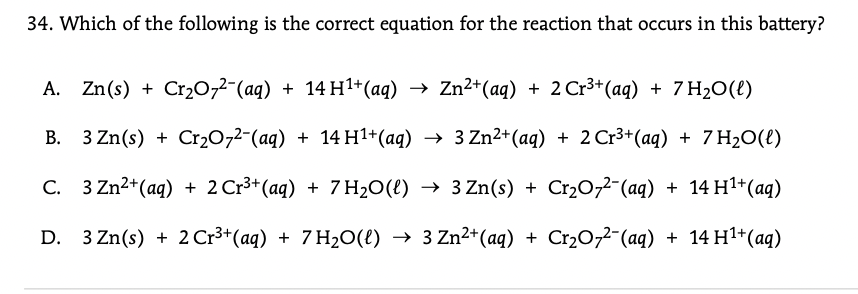
Transcribed Image Text:34. Which of the following is the correct equation for the reaction that occurs in this battery?
A. Zn(s) + Cr20,2-(aq) + 14 H1+(aq) → Zn²*(aq) + 2 Cr3*(aq) + 7 H2O(t)
B. 3 Zn(s) +
Cr20,2-(aq) + 14 H1+(aq) → 3 Zn2+(aq) + 2 Cr3+(aq) + 7 H2O(t)
C. 3 Zn2+(aq) + 2 Cr3+ (aq) + 7 H2O(t) → 3 Zn(s) + Cr2077¯(aq) + 14 H1+(aq)
D. 3 Zn(s) + 2 Cr3+(aq) + 7 H20(e) → 3 Zn²+(aq) + Cr2O,2-(aq) + 14 H1+(aq)
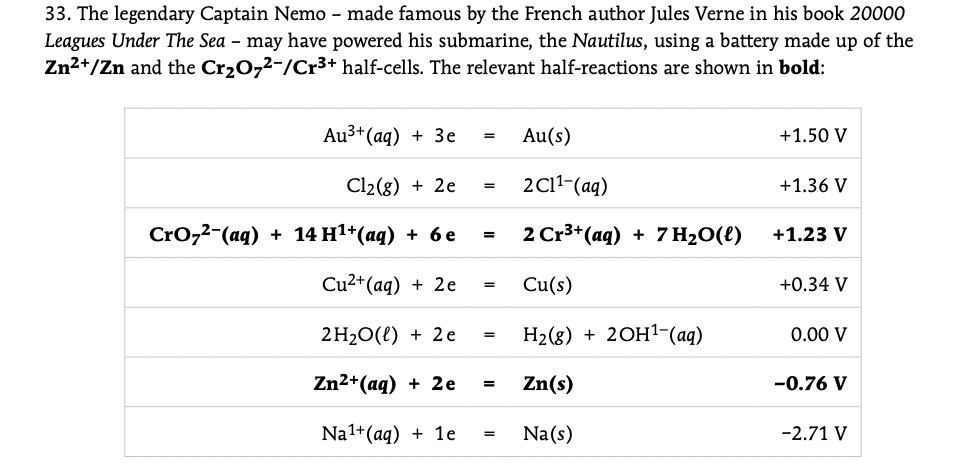
Transcribed Image Text:33. The legendary Captain Nemo - made famous by the French author Jules Verne in his book 20000
Leagues Under The Sea – may have powered his submarine, the Nautilus, using a battery made up of the
Zn2+/Zn and the Cr20,2-/Cr3+ half-cells. The relevant half-reactions are shown in bold:
Au3* (aq) + Зе
Au(s)
+1.50 V
Cl2(g) + 2e
2C11-(aq)
+1.36 V
Croz2-(ag) + 14 H1*(аq) + 6е
2 Cr3+(aq) + 7 H2O(l)
+1.23 V
=
Cu2+(aq) + 2e
Cu(s)
+0.34 V
2H20(l) + 2e
Н2(8) + 20H1-(aq)
0.00 V
Zn2+(aq) + 2e
Zn(s)
-0.76 V
=
Na1+(aq) + 1e
Na(s)
-2.71 V
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning