35. The starting point (X) on the graph represents a person who had been drinking water ad libitum. Which of the following lettered arrows indicates the change that will occur 60 minutes after drinking 1 L of distilled water? OA) 12001 Urine osmolality mOsm/Kg H₂0 900- A 600- 300- SO B) B Urine flow rate mL/min OC) OD) 10 OE)
35. The starting point (X) on the graph represents a person who had been drinking water ad libitum. Which of the following lettered arrows indicates the change that will occur 60 minutes after drinking 1 L of distilled water? OA) 12001 Urine osmolality mOsm/Kg H₂0 900- A 600- 300- SO B) B Urine flow rate mL/min OC) OD) 10 OE)
Macroscale and Microscale Organic Experiments
7th Edition
ISBN:9781305577190
Author:Kenneth L. Williamson, Katherine M. Masters
Publisher:Kenneth L. Williamson, Katherine M. Masters
Chapter45: Synthesis And Bioassay Of Sulfanilamide And Derivatives
Section: Chapter Questions
Problem 7Q
Related questions
Question
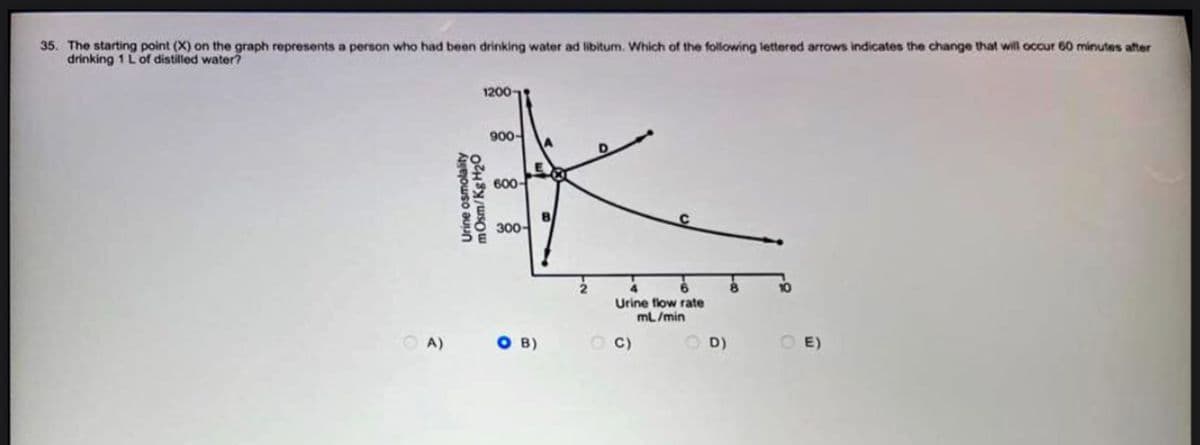
Transcribed Image Text:35. The starting point (X) on the graph represents a person who had been drinking water ad libitum. Which of the following lettered arrows indicates the change that will occur 60 minutes after
drinking 1 L of distilled water?
A)
1200
Urine osmolality
mOsm/Kg H₂O
900-
600-
300-
A
E
COT
SO B)
Urine flow rate
mL/min
C)
D)
E)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning