39. An enclosed mixture has a mass of 12.69723±0.00003 g, and after a chemical change occurs the mixture has a mass of 12.69724±0.00003 g. These results show that (A)the law of conservation of matter is not always true. (B)the law of conservation of mass is not always true. (C)the mass of the enclosed mixture remains constant within the experimental error of the measurement. (D)the mass of the enclosed mixture does not change. (E)the mass of the enclosed mixture increased.
39. An enclosed mixture has a mass of 12.69723±0.00003 g, and after a chemical change occurs the mixture has a mass of 12.69724±0.00003 g. These results show that (A)the law of conservation of matter is not always true. (B)the law of conservation of mass is not always true. (C)the mass of the enclosed mixture remains constant within the experimental error of the measurement. (D)the mass of the enclosed mixture does not change. (E)the mass of the enclosed mixture increased.
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter3: Matter
Section: Chapter Questions
Problem 5CR
Related questions
Question
I need help with questions 39-40? Could you explain which option is correct for each question?
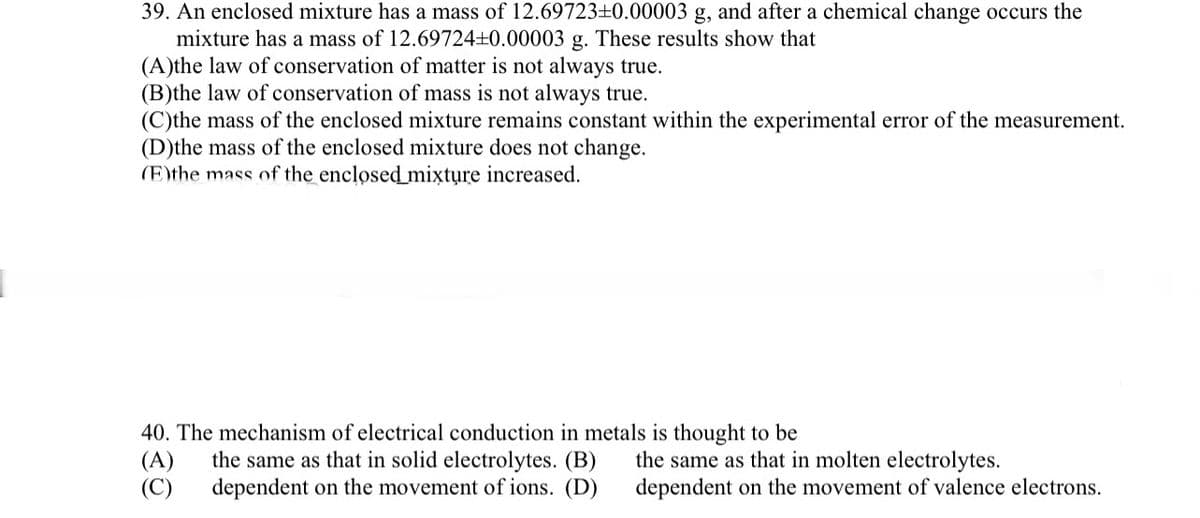
Transcribed Image Text:39. An enclosed mixture has a mass of 12.69723±0.00003 g, and after a chemical change occurs the
mixture has a mass of 12.69724±0.00003 g. These results show that
(A)the law of conservation of matter is not always true.
(B)the law of conservation of mass is not always true.
(C)the mass of the enclosed mixture remains constant within the experimental error of the measurement.
(D)the mass of the enclosed mixture does not change.
(E)the mass of the enclosed mixture increased.
40. The mechanism of electrical conduction in metals is thought to be
(A)
the same as that in solid electrolytes. (B)
(C) dependent on the movement of ions. (D)
the same as that in molten electrolytes.
dependent on the movement of valence electrons.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning