4-3. A liquid-phase chemical reaction A takes place in a well-stirred tank. The concentration of A in the feed is CAo (mol/m³), and that in the tank and outlet stream is CA (mol/m³). Neither concentration varies with time. The volume of the tank contents is V(m³) and the volumetric flow rate of the inlet and outlet streams is V (m3/s). The reaction rate (the rate at which A is consumed by reaction in the tank) is given by the expression r(mol A consumed/s) = kVC, where k is a constant. a. Is this process continuous, batch, or semibatch? Is it transient or steady-state? b. What would you expect the reactant concentration C, to equal if k = 0 (no reaction)? What should it approach if k → o (infinitely rapid reaction)? c. Write a differential balance on A, stating which terms in the general balance equation (accumulation = input + generation – output – consumption) you discarded and why you discarded them. Use the balance to derive the following relation between the inlet and outlet reactant concentrations: CA0 CA 1+ kV/V Verify that this relation predicts the results in Part (b).
4-3. A liquid-phase chemical reaction A takes place in a well-stirred tank. The concentration of A in the feed is CAo (mol/m³), and that in the tank and outlet stream is CA (mol/m³). Neither concentration varies with time. The volume of the tank contents is V(m³) and the volumetric flow rate of the inlet and outlet streams is V (m3/s). The reaction rate (the rate at which A is consumed by reaction in the tank) is given by the expression r(mol A consumed/s) = kVC, where k is a constant. a. Is this process continuous, batch, or semibatch? Is it transient or steady-state? b. What would you expect the reactant concentration C, to equal if k = 0 (no reaction)? What should it approach if k → o (infinitely rapid reaction)? c. Write a differential balance on A, stating which terms in the general balance equation (accumulation = input + generation – output – consumption) you discarded and why you discarded them. Use the balance to derive the following relation between the inlet and outlet reactant concentrations: CA0 CA 1+ kV/V Verify that this relation predicts the results in Part (b).
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Chapter1: Introduction
Section: Chapter Questions
Problem 1.1P
Related questions
Question
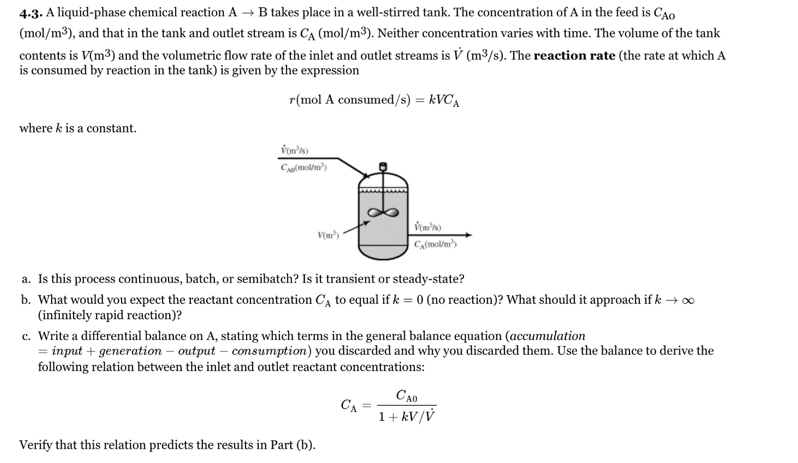
Transcribed Image Text:4-3. A liquid-phase chemical reaction A
takes place in a well-stirred tank. The concentration of A in the feed is CAo
(mol/m³), and that in the tank and outlet stream is CA (mol/m³). Neither concentration varies with time. The volume of the tank
contents is V(m³) and the volumetric flow rate of the inlet and outlet streams is V (m3/s). The reaction rate (the rate at which A
is consumed by reaction in the tank) is given by the expression
r(mol A consumed/s) = kVC,
where k is a constant.
a. Is this process continuous, batch, or semibatch? Is it transient or steady-state?
b. What would you expect the reactant concentration C, to equal if k = 0 (no reaction)? What should it approach if k → o
(infinitely rapid reaction)?
c. Write a differential balance on A, stating which terms in the general balance equation (accumulation
= input + generation – output – consumption) you discarded and why you discarded them. Use the balance to derive the
following relation between the inlet and outlet reactant concentrations:
CA0
CA
1+ kV/V
Verify that this relation predicts the results in Part (b).
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps with 7 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemical-engineering and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:
9781285061238
Author:
Lokensgard, Erik
Publisher:
Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:
9780072848236
Author:
Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:
McGraw-Hill Companies, The