Hydrogen is produced in the steam reforming of propane: CH«(g) + 3 H;O(v)– 3 CO(g) + 7 H:(g) The water-gas shift reaction also takes place in the reactor, leading to the formation of additional hydrogen: CO(g) + H;O(v) → CO:(2) + H;(g) The reaction is carried out over a nickel catalyst in the tubes of a shell-and-tube reactor. The feed to the reactor contains steam and propane in a 6:1 molar ratio at 125°C, and the products emerge at 800°C. The excess steam in the feed assures essentially complete consumption of the propane. Heat is added to the reaction mixture by passing a hot gas over the outside of the tubes that contain the catalyst. The gas is fed at 4.94 m³/mol C3Hs, entering the unit at 1400°C and 1 atm and leaving at 900'C. The unit may be considered adiabatic. Heating gas 1400°C, 1 atm сунне CyHule H,O) cố на но 125°c 800°c Spent heating gas 900°c | Calculate the molar composition of the product gas, assuming that the heat capacity of the heating gas is 0.040 kJ/(mo-°C).
Hydrogen is produced in the steam reforming of propane: CH«(g) + 3 H;O(v)– 3 CO(g) + 7 H:(g) The water-gas shift reaction also takes place in the reactor, leading to the formation of additional hydrogen: CO(g) + H;O(v) → CO:(2) + H;(g) The reaction is carried out over a nickel catalyst in the tubes of a shell-and-tube reactor. The feed to the reactor contains steam and propane in a 6:1 molar ratio at 125°C, and the products emerge at 800°C. The excess steam in the feed assures essentially complete consumption of the propane. Heat is added to the reaction mixture by passing a hot gas over the outside of the tubes that contain the catalyst. The gas is fed at 4.94 m³/mol C3Hs, entering the unit at 1400°C and 1 atm and leaving at 900'C. The unit may be considered adiabatic. Heating gas 1400°C, 1 atm сунне CyHule H,O) cố на но 125°c 800°c Spent heating gas 900°c | Calculate the molar composition of the product gas, assuming that the heat capacity of the heating gas is 0.040 kJ/(mo-°C).
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Chapter1: Introduction
Section: Chapter Questions
Problem 1.1P
Related questions
Question
100%
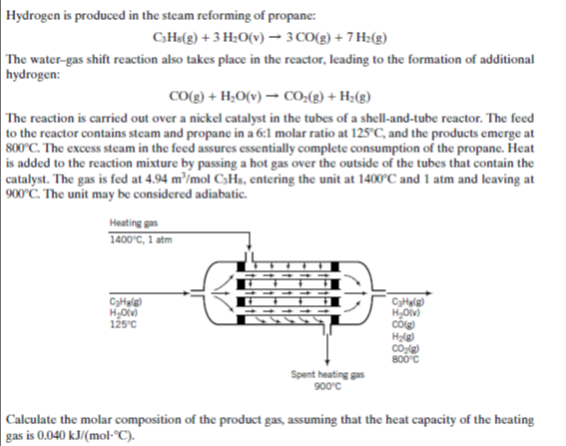
Transcribed Image Text:Hydrogen is produced in the steam reforming of propane:
CH«(g) + 3 H;O(v)– 3 CO(g) + 7 H:(g)
The water-gas shift reaction also takes place in the reactor, leading to the formation of additional
hydrogen:
CO(g) + H;O(v) → CO:(2) + H;(g)
The reaction is carried out over a nickel catalyst in the tubes of a shell-and-tube reactor. The feed
to the reactor contains steam and propane in a 6:1 molar ratio at 125°C, and the products emerge at
800°C. The excess steam in the feed assures essentially complete consumption of the propane. Heat
is added to the reaction mixture by passing a hot gas over the outside of the tubes that contain the
catalyst. The gas is fed at 4.94 m³/mol C3Hs, entering the unit at 1400°C and 1 atm and leaving at
900'C. The unit may be considered adiabatic.
Heating gas
1400°C, 1 atm
сунне
CyHule
H,O)
cố
на
но
125°c
800°c
Spent heating gas
900°c
| Calculate the molar composition of the product gas, assuming that the heat capacity of the heating
gas is 0.040 kJ/(mo-°C).
Expert Solution
Trending now
This is a popular solution!
Step by step
Solved in 10 steps with 12 images

Recommended textbooks for you

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:
9781285061238
Author:
Lokensgard, Erik
Publisher:
Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:
9780072848236
Author:
Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:
McGraw-Hill Companies, The