4) Consider a substance that undergoes a phase transition at To=300K and pressure Po=5bar. The Gibbs free energy per mole at constant pressure and temperature near the transition is shown below. G (kJ/mol) a) What type of phase transition is 600 this? Why? 400 200 P (bar) 4 10 b) From the figure, estimate the difference in volume AV between the two phases.
4) Consider a substance that undergoes a phase transition at To=300K and pressure Po=5bar. The Gibbs free energy per mole at constant pressure and temperature near the transition is shown below. G (kJ/mol) a) What type of phase transition is 600 this? Why? 400 200 P (bar) 4 10 b) From the figure, estimate the difference in volume AV between the two phases.
Related questions
Question
100%
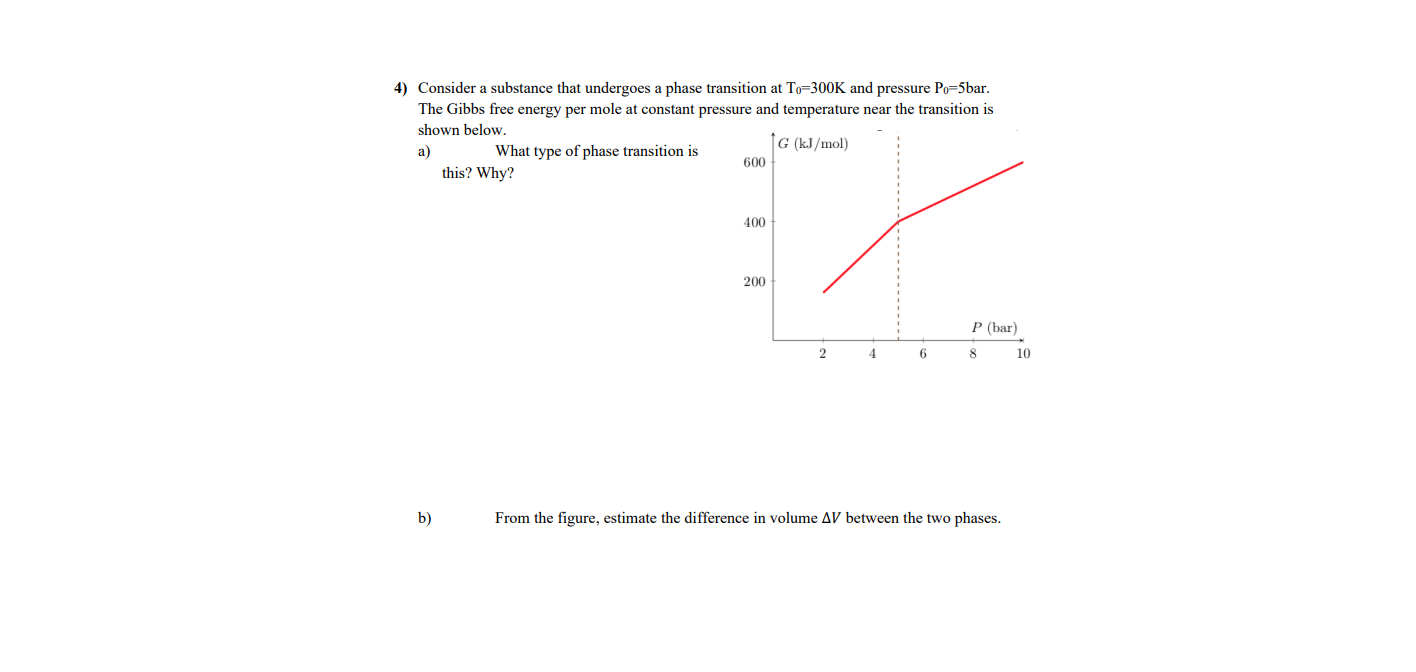
Transcribed Image Text:4) Consider a substance that undergoes a phase transition at To=300K and pressure Po=5bar.
The Gibbs free energy per mole at constant pressure and temperature near the transition is
shown below.
G (kJ/mol)
a)
What type of phase transition is
600
this? Why?
400
200
P (bar)
4
10
b)
From the figure, estimate the difference in volume AV between the two phases.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 4 images
